-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2021; 11(4): 66-80
doi:10.5923/j.chemistry.20211104.02
Received: Nov. 5, 2021; Accepted: Nov. 20, 2021; Published: Dec. 15, 2021

Geographical Prediction of Parsley Origins Based on Chromatographic Fingerprinting and Quantitative Analysis: Application to Mediterranean Cultivars
Moayyad Al-Hyasat1, Ramia Al Bakain1, Jérôme Vial2, Didier Thiebaut2
1Department of Chemistry, School of Science, The University of Jordan, Amman, Jordan
2Laboratoire Sciences Analytiques, Bioanalytiques et Miniaturisation, UMR 8231, ESPCI Paris, PSL Research University, CNRS, Paris, France
Correspondence to: Ramia Al Bakain, Department of Chemistry, School of Science, The University of Jordan, Amman, Jordan.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this study, for the first time, quantitative and qualitative chromatographic analysis for the content measurements of parsley varieties in nine cities of three big producers/consumers countries in the Mediterranean region: France, Lebanon and Jordan, were carried out. Parsley oils were extracted by hydrodistillation then examined by GC-MS to reveal their complex chemical profiles for plants clustering. Results showed that 29 compounds were quantified and identified in all parsley plant oil extracts. Among organics; apiol, myristicin, adamantane and benzenamine,N,N-4-trimethyl, not only displayed the highest levels in overall contents, but also were the responsible compounds for parsley-origin classification. Among the countries, France has clearly distinct content of benzenamine,N,N-4-trimethyl. GC-MS data were exposed to PCA and HCA to reveal: the variations of parsley chemical profiles as a result of geographical areas, to show the compounds that were responsible for clustering cultivars, and finally, to facilitate the future profile prediction and countries clustering of unknown parsley based on their chemical profile. Nine different cities of parsley samples from different origins were divided into two clusters based on benzenamine,N,N,4-trimethyl, apiol, myristicin, adamantane contents. Classification of parsley based on GC-MS profile will meet the practical requirements of parsley applications in clinical researches, industrial production, patients’ self-production, and contribute to the standardization of commercially available parsley cultivars in the Mediterranean countries. The results of this work will accelerate the future profile prediction and origin- clustering of unknown parsley based on their profile.
Keywords: Medicinal parsley, Gas Chromatography, Principal Component Analysis, Cluster Analysis, Geographical Prediction
Cite this paper: Moayyad Al-Hyasat, Ramia Al Bakain, Jérôme Vial, Didier Thiebaut, Geographical Prediction of Parsley Origins Based on Chromatographic Fingerprinting and Quantitative Analysis: Application to Mediterranean Cultivars, American Journal of Chemistry, Vol. 11 No. 4, 2021, pp. 66-80. doi: 10.5923/j.chemistry.20211104.02.
Article Outline
1. Introduction
- The huge amount of data in natural products science has been increased over the last decade due to the application of modern instruments and chemometrics. This development performed a better understanding in the chemistry of natural products, especially for those used in medical applications [1]. Using natural products, especially herbal plants as traditional medicine and their molecules with therapeutic ability have been extended worldwide [2]. The World Health Organization (WHO) classified the traditional medicine as a complementary or alternative [3] since this medicine has preserve its popularity in the developing countries and its use is rapidly distributing in industrialized regions [4]. The essential oils extracted from herbal plants contain different kinds of volatile compounds such as phenol derived aromatic and aliphatic, mono and sesquiterpenes compounds [5]. Different studies show that many essential oils and extracts obtained from plants are widely produced in the Mediterranean region [6]. Different varieties and large number of herbal plants are grown in the Mediterranean, for example; sage, savory, thyme, lavender, clove, oregano, rosemary, salvia judaica boiss [7-8], salvia fruticosa, origanum syriacum, melissa officinalis, althaea damascena, cyclotrichium origanifolium, origanum ehrenbergii, viola libanotica, clinopodium libanoticum, thymbra spicata and satureja thymbra [9-13], chamomile, menthe, achillea, artemisia, coriander and parsley. These plants showed their benefits in the biological activity tests for their antioxidant power, inhibitors to the growth of cancer cells [14], antispasmodic, antiemetic, stimulant, analgesic, and emmenagogue [15]. In addition to the medical usage, these essential compounds are used in food, drugs and perfumery industries [16]. For examples, in France, Lebanon and Jordan, many studies showed that herbal plants are used in cosmetic, food, pharmaceutical and perfumery applications, treatment of abdominal pains and disorders, flatulence, diabetes, common cold, arthritis, parasitic worms, neurodegenerative diseases, urinary tract infections and kidney stones [9-10,13,17-20].One of the most famous herbs produced and consumed in the Mediterranean Region is parsley (Petroselinum crispum) [21]. Parsley is a leafy, green herb, aromatic, culinary, and medicinal plant [22]. There are three types of parsley: the curly leaf parsley (P. crispum var. crispum) that is frequently used as a trimming; the flat leaf parsley (P. crispum var. neapolitanum) which is used in Mediterranean dishes such as tabbouleh and salad decoration; and the third kind is called root parsley (P. crispum var. tuberosum) that is developed as a root vegetable [23]. Fresh parsley is considered as a rich source of vitamin B, vitamin C, β-carotene, zinc, boron, fluorine, iron and calcium in an absorbable form [24]. Parsley is grown wildly outdoors and it is seasonally harvested in the Mediterranean, Middle East and North Africa (MENA) regions. The perfect environmental conditions to parsley growth are sunny seasons, soil humidity, and pH between 5.3 and 7.3 [24]. Parsley normal average height is 60 -120 cm and its chemical composition mainly depends on water stress, so that, it must be provided with continuously water source to get the highest quality and maximum amount [24]. Great differences reveal in the chemical composition of essential oils and the quality obtained from the parsley plant from different origins, and these differences are related to many factors: plant quality, geographical location, soil type, day length, irradiance, water supply, season of harvesting, climatic and soil properties, cultivation process, distillation technique and rain levels [6,25]. Regarding the extraction techniques of essential oils [19,26-33]; hydrodistillation by Clevenger type apparatus [26], liquid–liquid extraction [27], solvent extraction [28], solid phase micro extraction [32], ultrasound extraction [19] and microwave [33] have been implemented to extract the maximum amount of essential oils from plant. However, hydrodistillation by Clevenger type apparatus is the most famous technique since it is fast and showed high efficient extraction yield with low cost [26]. Regarding the analysis, GC-MS is the most popular technique used for analyzing the essential oils since it could separate complex mixtures into their individual components, identify and quantify them easily [25,34].Hierarchical cluster analysis (HCA) and Principal component analysis (PCA) are beneficial tools in analytical chemistry for data classifications. They were implemented in analytical studies for many purposes; to fingerprint the most relevant compounds in recognizing plant varieties, to detect the variation on chemical profiles as a result of growing plants in different areas and with variations in growth time, to ensure whether the cultivars in the cluster analysis could also be grouped together and to show the compounds that were responsible for grouping cultivars between clusters [35]. There is currently no available systematic clustering for the parsley-origin samples between Mediterranean countries that are considered as big cultivars and consumers, which is indispensable to explore the similarities/differences if any between plants-origin batches. Hence, this study was implemented to examine the chemical profiles of essential oils in parsley grown in Mediterranean countries. For the first time, parsley plants obtained from nine cities of three countries; France, Jordan and Lebanon were collected, extracted and analyzed using GC-MS. The parsley samples were scanned to reveal all possible compounds necessary for plants clustering. The validated method was evaluated for selectivity and precision (repeatability and day to day intermediate precision). Grouping the plants samples from different countries was accomplished using unsupervised clustering methods (i.e. PCA and HCA). Furthermore, the importance of the chemical contents for samples classification has been revealed. To the best of our knowledge, this study is the first to classify the Mediterranean parsley samples and to use the PCA and HCA to predict the location for future samples analyzed in blind.
2. Material and Method
2.1. Parsley Samples
- Fresh parsley plants were collected from local cultivators during August/September 2019. Samples were selected from three countries France, Jordan and Lebanon. From each country, three different cities were selected to collect parsley from their cultivators, and from each city; 5 bundles of parsley were taken. French cultivated samples were obtained from the following areas and regions: Paris (F1), Dijon (F2) and Picardie (F3), the Jordanian samples were provided from Southern Jordan Valley (J1), Amman airport region (J2) and Mafraq (J3), and finally the Lebanese ones were collected from Tripoli (L1), Beka'a Valley (L2) and Kherbet Eljord (L3).
2.2. Reagents
- The chemicals used in this work were of HPLC ultra-grade: n-Hexane 95% obtained from TEDIA, USA, and sodium sulphate anhydrous Na2SO4 were purchased from Biochem Chemopharma, France. Ultra pure water (18 MΩ cm-1) was produced by Milli-Q plus water purification instrument (Millipore, Molsheim, France).
2.3. Essential Oils Extraction from Parsley
- The extraction of parsley essential oils was carried out by hydrodistillation using a Clevenger type apparatus according to the following procedure: after collecting the plants samples from the cultivars, the leaves of the 5 bundles had been cut down by plant scissors to ensure a homogenous mixture of leaf particles with no seeds or stem, and inserted into stainless steel blender container. Then, 1.5 L of ultra pure water had been added. The contents had been mixed for about 15 min, after that, immediately, the mixture was transferred into 5.0 L Clevenger apparatus (Chitransh borosilicate, India). Finally, the mixture was heated under temperature regulation. After the operating temperature reached the boiling point of the mixture in the Clevenger apparatus distillation flask, the heating was continued for extra 2 hrs. Parsley essential oils were collected in 10.0 mL beaker, and then a small amount of anhydrous sodium sulphate was added to eliminate any remaining water in the extracted oil. A concentration of 0.02% (v/v) extracted essential oil/ n-hexane was prepared for the GC-MS injection. Finally, Acrodisc syringe (PAU-Gelman Lab, 0.45 μm, 25 mm diameter) membrane micro-filter was used to filter the extract, and then the extract was collected in a screw-capped vial. Samples were reserved in the dark in a freezer (at least -10°C) until time of analysis. The extractions and the GC-MS injections were carried out in triplicate. The injection volume was 10.0 μL. The content of each essential oil was reported as an average of the three injections. Quantification of parsley chemical content for each compound was achieved by internal normalization, i.e. by comparing the ratio of peak area of the component in the sample with the summation of all peak areas in the parsley plant sample.
2.4. Gas Chromatography – Mass Spectrometer Detector
- The chemical profiles of the parsley extracts obtained by GC-MS were all generated using a VARIAN Model CP-3800 Gas Chromatographic system coupled with Saturn 2200 GC/MS/MS, CP-8400 autosampler. The GC column was SLB-5MS fused silica capillary column, 30 m length × 0.25 mm internal diameter, 0.25-µm film thickness, (Sigma-Aldrich, USA). The front injector temperature was set at 250°C.The temperature was programmed in a linear generic gradient mode from 60°C to 250°C at a rate of 3°C/min with a holding time of 1 min. The carrier gas was helium of (99.999% purity) at constant column flow rate of 1.0 mL.min-1. Solutes detection was achieved using mass spectrometer with scan range from 40-450 m/z, and samples were injected in a split ratio of 1:10 with ionization energy of 70 eV. The overall runtime was 65 min/sample, and the injection volume was 10.0 μL/sample.
2.5. Chromatographic Identification and Quantification of Extracted Essential Oils
- To evaluate the selectivity, solvent blank was injected to ensure that no artifact signal peaks were appeared at the targeted retention time. The quantitative analysis for each essential oil component expressed in a content% was calculated according to the following estimation:
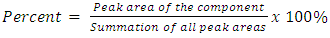 | (1) |
3. Statistical Analysis
3.1. Multivariate Analysis of Parsley Samples Using Unsupervised Clustering Methods
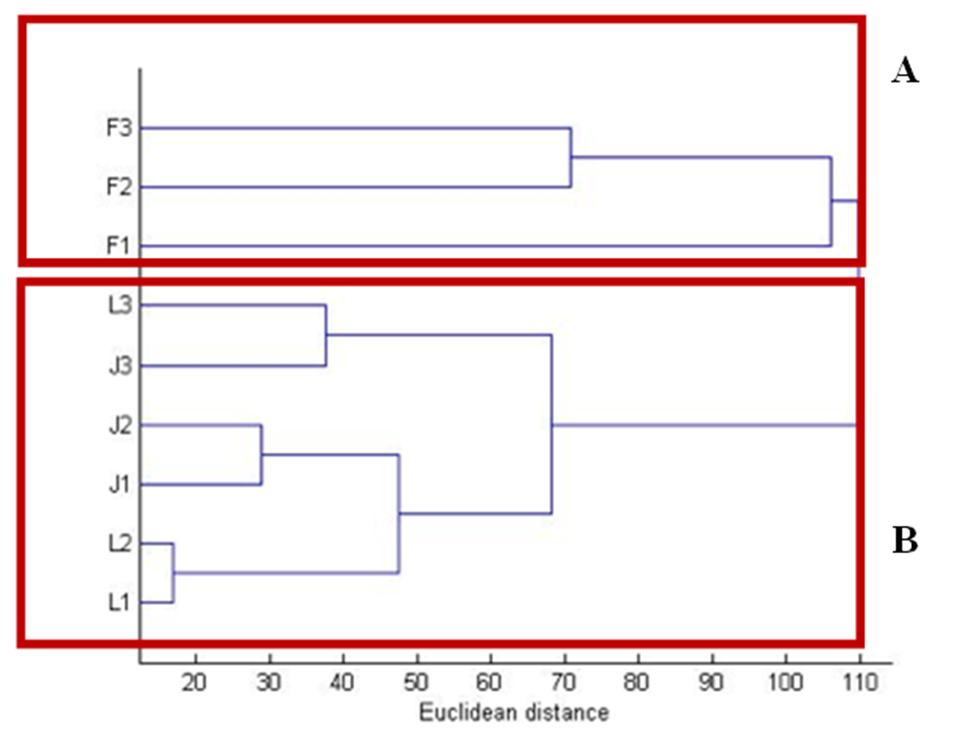
- Arrangement of chromatographic dataAt first, the GC data was organized in a data matrix Xn×l, where n is the number of plants (i.e. cities) and l is the number of solutes. In this study, matrix X had the size of 9×29. Matrix X was subjected to clustering methods (as will be explained below). All the collected samples were priori assigned to class membership; hence, unsupervised clustering methods will be applied [35-36]. PCA and HCAThe most common unsupervised methods in analytical chemistry for grouping and clustering samples are PCA and HCA [37]. In this study, HCA and PCA were implemented to confirm whether the parsley obtained from different cities would be grouped together based on the GC-MS analysis outcomes of the 29 compounds in each plant sample. In fact, PCA reveals the compounds that are responsible for grouping the samples [35,38-39]. The classification outcomes might be necessary for clinical researchers in the selection and discrimination of plant samples in certain cities. The results may help to show the similarities and the differences between parsley profiles cross the big producers and consumers of parsley (i.e. Lebanon, Jordan and France). In PCA, matrix X is divided into two matrices; score matrix T and loading matrix L using non-linear iterative partial least-squares or singular value decomposition. Therefore, X9×29 is divided into T9×h and Lh×29 (h is the number of optimum factors required for matrix decomposition). The value of h is estimated by leave-one-out cross validation method. When h is computed, scores and loadings vectors are appeared to estimate the clusters and the significance of variables for clustering plants. Using HCA in GC data is important to emphasize their natural clusters and patterns in a two dimensional space. The results are showed in the form of a dendrogram to allow rapid figure out of clusters and correlations between the studied samples [36,38-39].
3.2. Statistical Software
- Data acquisition and processing were achieved using MS Workstation software (version 6.6 SP1). PCA and HCA statistical analysis were performed by Chemoface 1.61 software which work under Matlab® (Mathworks, 8.6, USA) and XLSTAT software (Excel, Microsoft®). The peak area ratio was measured for each solute as following: peak ratio = peak area of solute /summation of peak areas of the sample) and used to quantify the content of the each solute.
4. Results and Discussion
4.1. GC Analysis and Variability of Parsley Contents in Different Samples
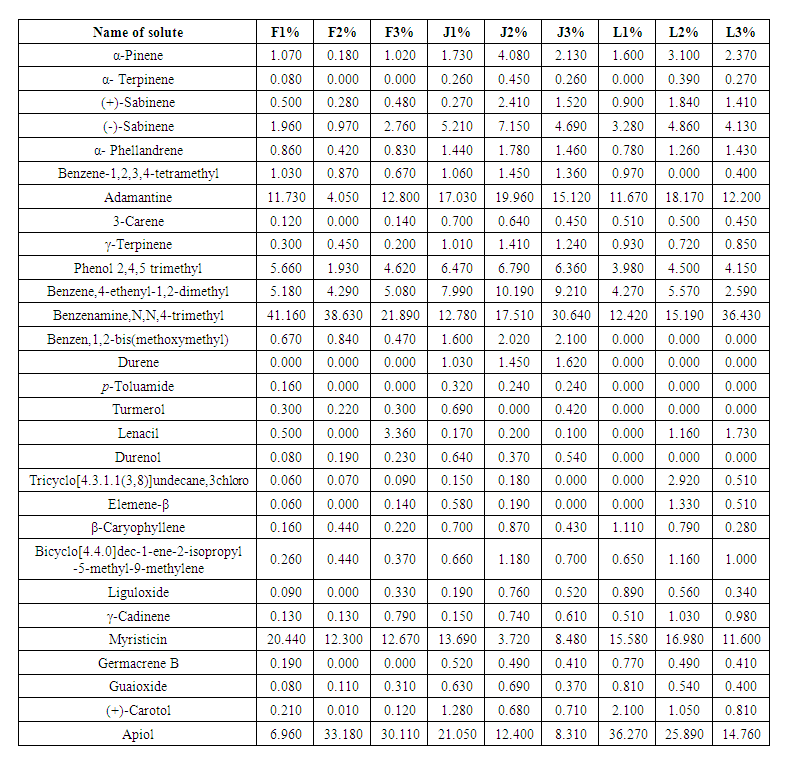
- Twenty nine compounds were detected in parsley samples in this study, and identified in the plant samples over the 9 cities in Lebanon, Jordan and France as shown in Table 1.
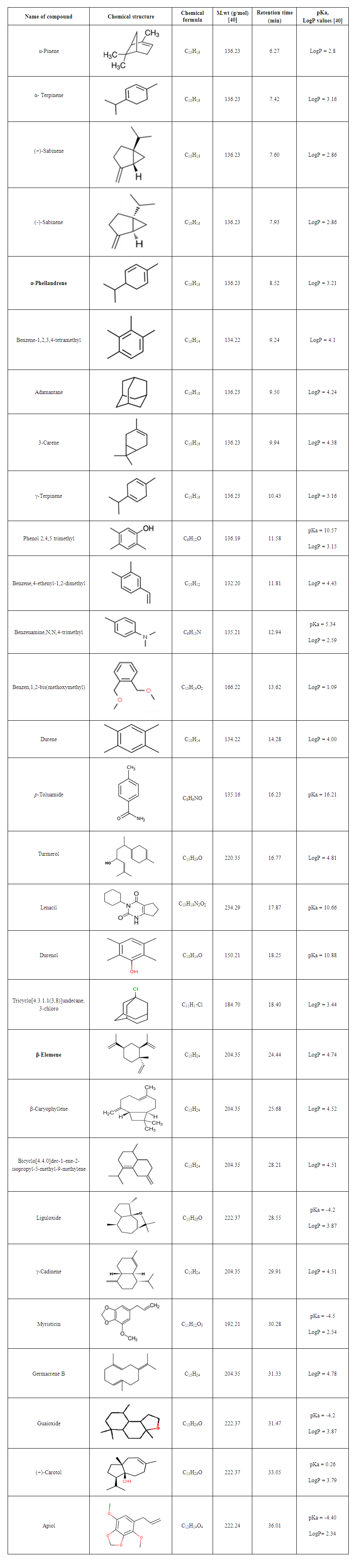 | Table 1. The 29 solutes identified in parsley samples obtained from the nine cities in France, Jordan and Lebanon |
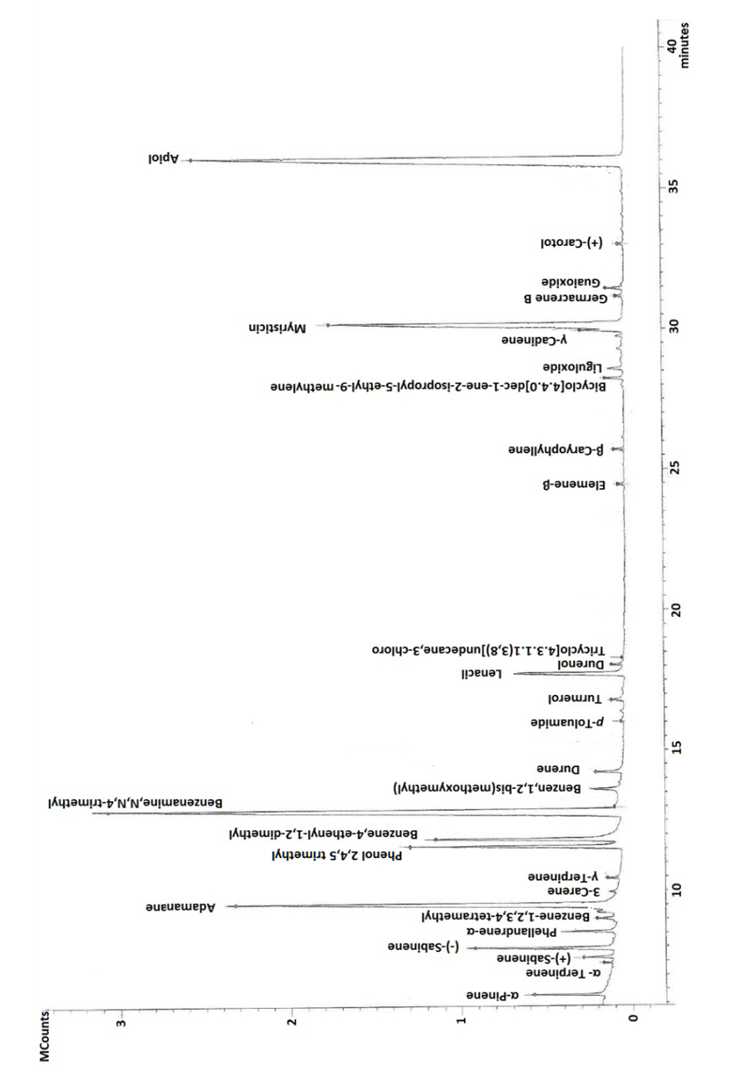 | Figure 1. GC-MS of Picardy-origin/France parsley |
4.2. Comparison between Parsley Chemical Contents of French, Jordanian and Lebanese Samples
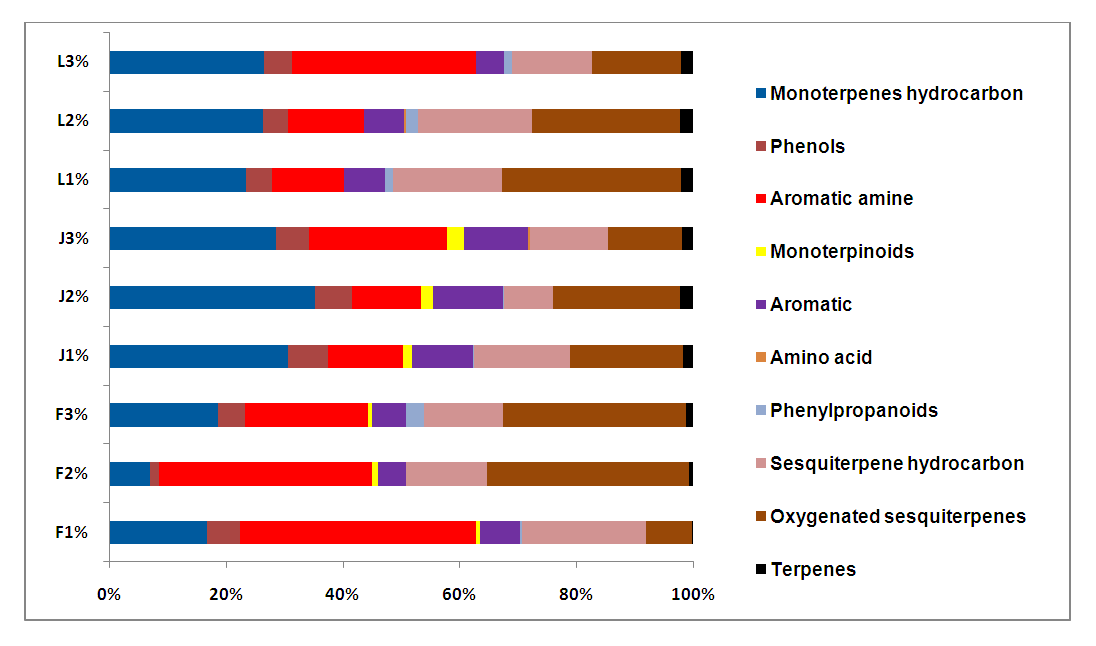
- It was clear that all parsley plant samples collected from the 9 areas have the same major four abundant components: adamantane, benzenamine,N,N-4-trimethyl, myristicin and apiol, with different order from one sample to another. There are variations between one content to another in the same sample, this result is referred to overlapping or interfering of other peaks due to some physical changes like solubilization and co-dissolution in the process of decoction when heat is applied to distillate essential oil compositions [44].Our results were in agreement with other studies from Spain, Egypt, Brazil and Saudi Arabia which found that both myristicin and apiol were the major components [45-47]. Adamantane was also mentioned in previous study from Turkey as a main component [48], where, benzenamine,N,N-4-trimethyl was not found in the parsley from Spain, Egypt, Turkey, Brazil and Saudi Arabia [45-48], which provide the Mediteranian parsley a special characteristic to facilitate distinguishing it from other parsley-type origin.A quick look at the distribution of the compounds in our study showed retention time was mainly affected by the volatility of compounds. Any peak before 6 min was mainly the solvent n-hexane which is very volatile. The compounds detected between 6-10 min had the highest volatility (α-pinene, α-terpinene, (+) sabinene, (-) sabinene, α-phellandrene, benzene-,1,2,3,4-tetramethyl, adamantane, 3-carene). Compounds identified between 10-20 min were moderately volatile (γ-terpinene, phenol 2,4,5 trimethyl, benzene,4-ethenyl-1,2-dimethyl, benzeneamine,N,N,4-trimethyl, benzene,1,2-bis(methoxymethyl, durene, ρ-toluamide, turmerol, lenacil, durenol, tricyclo[4.3.1.1(3,8)]undecane,3-chloro). Finally, late detected compounds had the lowest volatility (β-elemene, β-caryophyllene, bicyclo[4.4.0]dec-1-ene-2-isopropyl-5-methyl-9-methylene, liguloxide, γ-cadinene, myristicin, germacrene, guaioxide, (+)-carotol and apiol). Regarding the three Mediterranean countries in this study, in general, the same compounds showed the highest, modest and lowest concentration, which means that they have similar content for the main compounds regardless the small differences in their level.The quantitative and qualitative results showed variations in the percentage at each compound; this is related to: 1) geographic variations; climate, seasonal changes, amount of time of exposure to sunlight during the day, humidity, 2) environmental variations; soil composition, pH of the soil, 3) type of original seeds of plant [35,49]. Jordan and Lebanon share common temperature’s variations during the four seasons of the year with main differences in humidity percentage (i.e. humidity in the Eastern Mediterranean side of Lebanon is high due to the topography of the narrow coastal plain close to a long parallel and high mountains chain). The evaporation from the sea, return back to the crowded cities of the plain producing humidity up to 85% between July-September. Where France has different temperature variations during the year (lower temperature than Jordan and Lebanon during the whole year, especially in summer and spring seasons). Moreover, chemical variation could be explained to genetic factors impacting the secondary metabolism, harvesting time of the biological material in different development phases, interaction with microorganisms and insects, and different post-harvest techniques [45,50]. In addition, the growth of parsley in hot weathers such as Mediterranean regions (in late spring and summer), make it easily open to stress because of water deficiency in warm countries. This stress causes a decrease in biomass, leaf number per plant that resulted from a disruption of photosynthesis, transpiration and metabolic processes [41,51]. The effect of water stress is not only impacted the plant growth and essential oil yield, but it may change the quality of the oil. To unravel the differences/similarities between parsley samples from France, Jordan and Lebanon, multivariate analysis has been applied to the chromatographic data in the next section.
4.3. Parsley Characterization by PCA and HCA
- Both PCA and HCA are performed to confirm whether the parsley obtained from different cities would be grouped together based on the GC-MS measured active ingredients, including volatile oils with a total number of 29 solutes (29 detected peak). PCA can reveal the compounds that are responsible in grouping parsley samples. Parsley classification will be helpful in the industrial production, and informative map for individual cultivars. Indeed, these results would help to show the similarities and the differences between parsley-origin samples of some Mediterranean countries that are considered as big producers and consumers of parsley.Arrangement of chromatographic data: Chromatographic data can be arranged as a data matrix X of n samples or location rows and l variables or compounds. In this study, matrix X has the size of 29 (solutes) × 9 cities. Matrix X was subjected into HCA and PCA as discussed below. Data was preprocessed using normalizing methodology which allowed for better and interpretably PCA outputs.Quantitative parsley classification by PCA and HCAIn this study, two data matrices where built. The data matrix X (29×9) (i.e. 29 solutes from 9 cities) is divided to two matrices, score matrix T and loading matrix L using PCA algorithm. In PCA at first, the loadings are computed. In math, loadings are the Eigen vector of the matrix (XXT) [37]. The loadings are grouped into a matrix L. The gathered loadings are orthogonal and normalized. The relationship between the original matrix X, the loading matrix L and the score matrix T is described as:
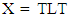 | (2) |
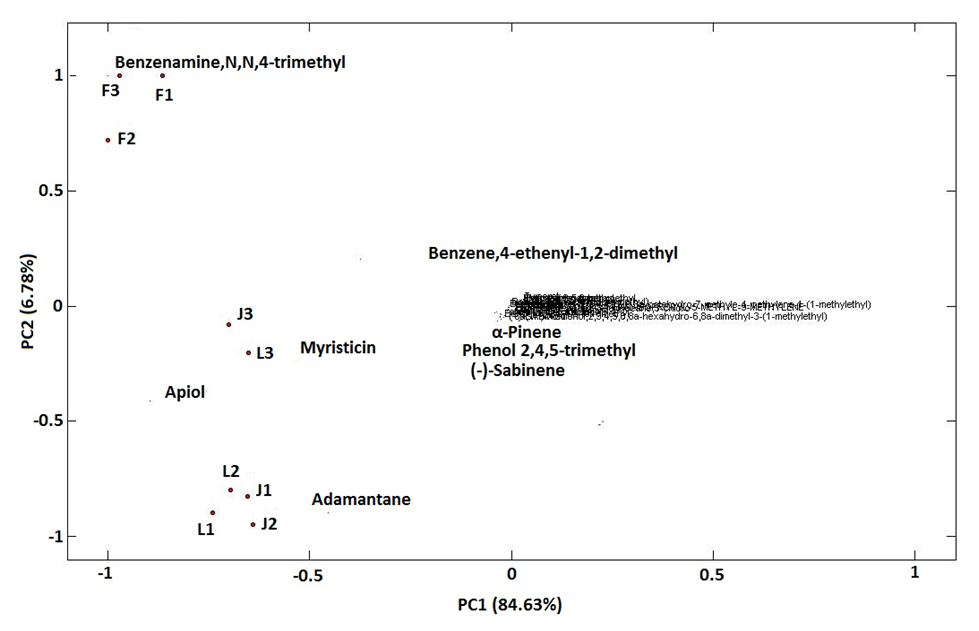 | Figure 4. PCA output: bi-plot obtained from the 9 origin- parsley oil components of France, Jordan and Lebanon |
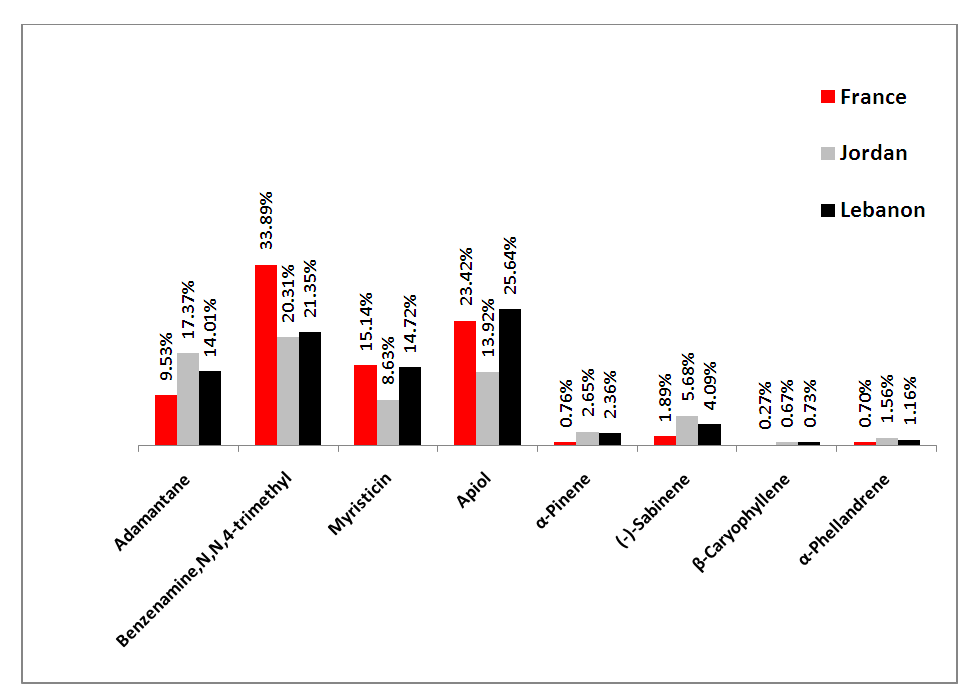 | Figure 5. Average abundance of the four main compounds selected by PCA model in the three Mediterranean countries samples in comparison to some other parsley contents |
5. Conclusions
- GC-MS was used to scan the chemical contents of 29 contents in 9 cities/regions of 3 Mediterranean countries: France, Jordan and Lebanon-parsley plants. The chemical contents were used to group parsley samples with the help of PCA and HCA. The grouping outputs uncovered the geographical origin of parsley plants. By HCA and PCA, the 9 parsley-origin plants were classified: first cluster consisted of the Lebanese and Jordanian cities, the second cluster had only the French plants that revealed distinct constituents of benzenamine,N,N-4-trimethyl. Multivariate analysis revealed also which constituents are major in differentiating cultivars since plants were clustered into 2 clusters; cluster A is benzenamine,N,N-4-trimethyl dominant, cluster B is apiol, myristicin and adamantane dominant. The results were fairly efficient since classifications based intensively on all contents considering all medically relevant compounds in parsley. Further studies will be carried out in the future to include parsley from all the Mediterranean countries in the same period in order to fill up the missing data in this area.
ACKNOWLEDGEMENTS
- Dr. Ramia Al Bakain and Moayyad Al-Hyasat gratefully acknowledge the financial support provided to this project by (1) L’Agence Universitaire de la Francophonie-Moyen Orient (AUF - Moyen Orient) and (2) Elsevier Foundation and 3) the University of Jordan. Indeed, great appreciation goes to Prof. Mervat El-Hoz, Dr. José Dugay and Mr. Ismail Abaza for their experimental help and technical support.
Conflict of Interests
- The authors declare that there are no conflicts of interest regarding the publication of this paper.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML