-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2021; 11(3): 49-51
doi:10.5923/j.chemistry.20211103.02
Received: Jul. 15, 2021; Accepted: Jul. 28, 2021; Published: Aug. 15, 2021

On the Chemistry of Sonnenschein Test for Strychnine
Francisco Sánchez-Viesca, Reina Gómez
Organic Chemistry Department, Faculty of Chemistry, National Autonomous University of Mexico, Mexico City (CDMX), Mexico
Correspondence to: Francisco Sánchez-Viesca, Organic Chemistry Department, Faculty of Chemistry, National Autonomous University of Mexico, Mexico City (CDMX), Mexico.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The study of the chemistry of a bioactive compound is important, the more if the substance is a violent poison. In this case chemical results are applied in forensic chemistry. The Sonnenschein test consists in the electron-transfer oxidation of strychnine, via cerium mediated radical reactions. Besides, this test produces a violet colour more stable than that obtained with potassium dichromate which is the recommended assay for identification of this drug. Since neither the chemistry nor the reaction mechanism have been advanced, we provide the electron flow, step by step, from the alkaloid to the complex oxidation product.
Keywords: Ceroceric oxide, Ceric sulphate, Radical reactions, Reaction mechanism, Reactive intermediates
Cite this paper: Francisco Sánchez-Viesca, Reina Gómez, On the Chemistry of Sonnenschein Test for Strychnine, American Journal of Chemistry, Vol. 11 No. 3, 2021, pp. 49-51. doi: 10.5923/j.chemistry.20211103.02.
1. Introduction
- The chemistry related to the rare –earth metal cerium is scant. However, Sonnenschein used ceroceric oxide and sulphuric acid for strychnine detection. This spot test produces an intense violet colour. In this communication, the reactions taking place in this test are described. It is a one-electron process and we arrived, step by step, to the complex oxidation product whose structure was elucidated decades after the test was proposed.This paper is a follow-up of other studies on reaction mechanism, [1-5].
2. Antecedents
- The test understudy is due to the German pharmacist Franz Leopold Sonnenschein (1819-1879). He used Ce3O4 and sulphuric acid for strychnine identification. He published his test in a medical, in a pharmaceutical, and in a chemical journal, [6-8]. His work was reviewed in England, [9], and registered in the United States, as indicated [6,7].He named the reagent Ceroxyduloxide (cerium oxydulus oxide) in reference to the lowest and to the highest oxidation states of cerium in this compound. Ce3O4 has been termed ceroceric oxide, [10], but it can be considered an oxysalt (2CeO.CeO2), cerium dioxide acting as anhydride in combination with cerium monoxide (ortho-salt), in a similar manner as in minium, Pb3O4.The Ce(II) compounds are scarce, but CeI2,Ce(OH)2 and CeO are known [11-13].The Sonnenschein test produces a blue colour more stable than that obtained with chromic acid, a method recorded in ‘Modern Plant Analysis’ and in ‘Drug Analysis’, [14,15].The blue colour gradually turns into cherry red and then remains unchanged for several days. In terms of sensitivity even 1 μg of strychnine can still be clearly recognized.The ceric ion is a strong oxidizer, especially under acidic conditions. When ceric compounds are reduced, cerous derivatives are formed and the orange colour of cerium(IV) ions vanishes since cerium(III) ions are colourless, [16,17].The electronic configuration of the rare-earth metal cerium is: [Xe] 4f15d16s2, [18]. These outer electrons are mis- sing in Ce+4.The electronic configuration of Cerium+3 is: [Xe] 4f1, [19].Even though Ce+4 presents a noble gas structure, it can be reduced to Ce+3 in order to decrease the positive charge in the nucleous. Compare Kossel, [20]. Therefore, it can accept one electron in 4f subshell.
3. Discussion
- Strychnine, Figure 1, is a seven-ring alkaloid and exhibits a lactam, a cyclic ether, a double bond, and a tertiary amine.
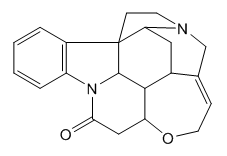 | Figure 1. Graphic formula of strychnine |
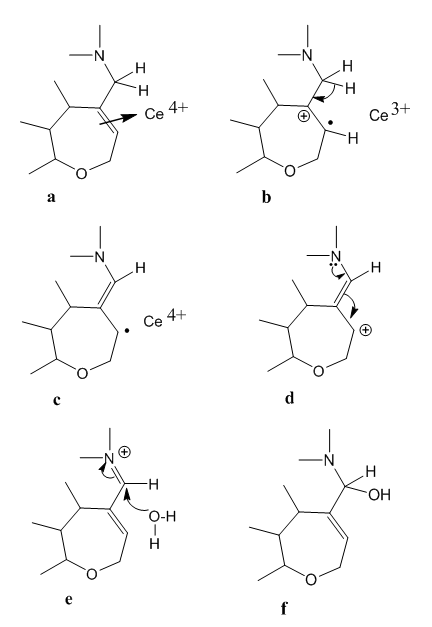 | Figure 2. First oxidation product of strychnine |
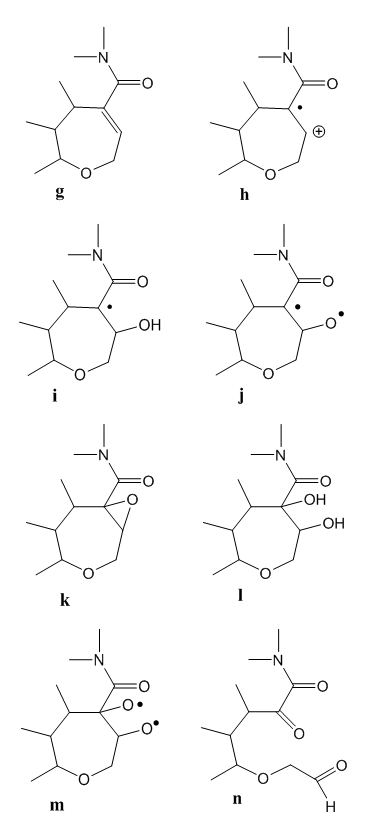 | Figure 3. Next oxidation steps of strychnine |
4. Conclusions
- We have studied the chemistry involved in the Sonnenschein test for strychnine identification. This redox process occurs via cerium mediated radical reactions. These are: electrophilic attack to the double bond, hydrogen elimination, free radical subtraction by cerium(IV), isomerization, alcohol oxidation, carbocation neutralization by water, epoxide formation, acid hydrolysis, ring opening, and aldehyde oxidation. Some reactions are repeated.This way the route from the alkaloid to the tetra-functional final product has been described. It is based on the reactivity of the groups present in strychnine, as well in the chemical deportment of the reagent.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML