-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2021; 11(2): 37-41
doi:10.5923/j.chemistry.20211102.03
Received: Mar. 24, 2021; Accepted: Apr. 23, 2021; Published: Apr. 30, 2021

Phthalate Esters in the Environment: Sources and Quantification
Adelagun R. O. A. 1, Kamba E. A. 1, Berezi E. P. 2, Aikhoje E. F. 1, Ngana O. C. 1, Muoneme B. O. 3
1Department of Chemical Sciences, Federal University Wukari, Taraba State, Nigeria
2Chemistry Department, Bayelsa Sate College of Education, Brass Island, Bayelsa State, Nigeria
3Department of Chemistry, Federal University of Agriculture, Markudi, Benue State, Nigeria
Correspondence to: Adelagun R. O. A. , Department of Chemical Sciences, Federal University Wukari, Taraba State, Nigeria.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Consequent upon their widespread use as plasticizers and high volume of production, phthalates constantly diffuse and release into the various environmental components (air, water, soil) has become noticeable. In this study, levels and presence of phthalate esters were analyzed in newly purchased plastic toys and in polyethylene terphthalate (PET) bottled drinking water samples. Phthalate esters (PEs) in the samples were liquid-liquid extracted, pre-concentrated and analyzed for detection and quantification using HPLC. From the data obtained, the levels of DMP, DEP and DBP in the PET drinking water samples did not exceed the stipulated threshold levels while the level of DEHP was dominant and exceeded the safe limit. PEs were detected in all the 10 plastic toys samples analyzed including mouthable ones (teethers) used by children, imported into the country from China, Taiwan, etc . The values obtained revealed that the concentrations of PEs in the plastic toys ranged between 0.96 – 532 (µg/l). Also the percentage (w/w) values obtained were significantly higher and ranged between1.96 - 79.88% than the European Union (EU) recommended limits for all phthalate esters in toys, this portends risk to children who innocently put these toys in their mouth or chew them, as the toxic chemicals could leach into their blood stream. These results can be used as reference levels for future monitoring programs for pollution studies.
Keywords: PET Drinking Water, Phthalate Esters, Plastic toys, Safety, Quantification
Cite this paper: Adelagun R. O. A. , Kamba E. A. , Berezi E. P. , Aikhoje E. F. , Ngana O. C. , Muoneme B. O. , Phthalate Esters in the Environment: Sources and Quantification, American Journal of Chemistry, Vol. 11 No. 2, 2021, pp. 37-41. doi: 10.5923/j.chemistry.20211102.03.
Article Outline
1. Introduction
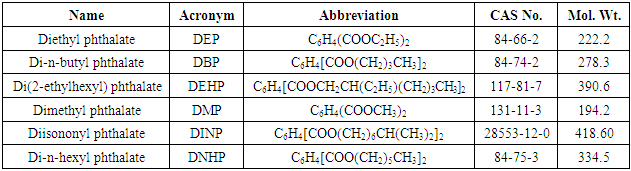
- Phthalate esters are the dialkyl or alkyl aryl esters of 1,2-benzenedicarboxylic acid (phthalic acid) produced by reacting phthalic anhydride with an appropriate alcohol (usually 6- to 13-carbon). They are colorless, odorless liquids with low water solubility, high oil solubility and low volatility (EPA, 2012). Phthalate esters are industrial chemicals used widely as plasticizers to impart flexibility and durability to polymers and plastics. Phthalates represent 69% of plasticizer used in USA, 92% in Western Europe and 81% in Japan [1].Table 1 contains some of the 18 commercial phthalate esters. Phthalates are important industrial chemicals used in a variety of applications. These include pharmaceuticals (either as inactive ingredients in producing enteric coatings or as part of the active ingredients) and personal-care products (e.g. perfume, eye shadow, moisturizer, nail polish, liquid soap, hair spray), building/household materials (e.g. shower curtains, vinyl upholstery, adhesives, floor tiles), printing inks and coatings, food products (food and drink containers and wrappers) and textiles. Also included are adhesives, paints, insect repellant, detergent, packaging and production of children's toys and other children's products such as chewy teethers, soft figures and inflatable toys [2-3]. PET is suitable for food packaging applications, especially for drinking because of its chemical inertness and physical properties.
|
2. Experimental
2.1. Samples Collection
- Five (5) different brands of commonly sold and consumed bottled water from randomly selected Super markets in Wukari, Taraba Sate, Nigeria was used for this study. All the water samples were packed in PET plastic bottles with High Density polyethylene (HDPE) plastics. Also, ten (10) new plastic toys samples for children (5 months-4 years) such as Teethers, Barbie dolls, baby suckers, Plastic trucks and Rabbits were purchased from Supermarkets in Wukari Local Government area, Taraba State, Nigeria.
2.2. Quality Control and Assurance
- Precautions were taken to avoid contaminations during sample collection and processing. It was ensured that no plastic equipment was used and all glasswares were precleaned. Also all equipments used were cleaned between samples and processing. All data were subjected to strict quality control procedures. Detergent was not used during sample processing. Quality assurance study was carried out in terms of Recovery Studies and Limit of Detection. The Recovery studies done for the PEs were carried out in order to ascertain the efficiency of extraction and analytical procedures adopted since there are no certified reference materials available. This was carried out by adopting the method stipulated by [20]. The Limit of Detection study carried out was to assess the lowest amount of analyte in sample; this was determined by adopting the method used by [21]. Identification was based on Retention time. Quantification of phthalate by internal standard was done by adopting the method of [21]. Analytical grade reagents of the PEs (DMP, DEP, DBP, DEHP) were purchased from Aldrich chemical company and Fluka AG, including the individual standard stock solution, and prepared in methanol.
2.3 Sample Pretreatment
- About 1.0 L of each water sample for the PEs analysis was spiked with the PEs recovery standard then extracted by Liquid-liquid extraction in a separatory funnel using 3 x 50 ml dichloromethane for 7 h. The combined solvents extracted were dried over anhydrous sodium sulphate and concentrated using a rotatory evaporator. Plastic sections of the PVC toys were cuts into pieces and pulverized into plastic powder. 10 g of each pulverized powder were weighed using an analytical balance (Mettler Toledo AB250) and Liquid-liquid extracted with soxhlet extractor using 3 x 50 ml dichloromethane for 7 h at 60°C.The combined solvents were dried in a glass chromatographic column (2.0 cm X 20 cm) packed with silica gel using anhydrous granular sodium sulphate. Hexane was used to elute the column for non-polar hydrocarbons and ethyl acetate was used to elute the column for phthalates prior to exposure to the Na2SO4 layer and concentrated in a thermostated water bath at 30°C. The residue was dissolved in 1 ml acetonitrile for instrumental analysis. Individual standard solutions of 100 mg/L of each of the phthalate standards and the internal standards were prepared by weighing accurately 1 mg of the standard into a 100 ml standard flask and made up to mark with the HPLC grade acetonitrile as solvent, from which standard mixtures of phthalates and internal standards were obtained. 20 µl of mixed standard solutions of the phthalates and the internal standards was added to the concentrated extract. The samples were transferred to a glass microvial for HPLC analysis.
2.4. Instrumental Analysis
- An AKTA tm Basic 10/100 HPLC (Amersham Pharmacia Biotech) coupled with UV detector transmitting at 254 nm was used. It comprises a controller compartment, CU-900 monitor; UV-900 Pump; P-900 Valve (INV-907), an auto-sampler (A-900) with a flow cell of 10 mm with elution capacity 1.0ml/min at pressure of 10Mpa. Chromatographic separation was carried out using a thermostatic capillary column C18 (S5OD52 250 cm X 3.0 mm) at ambient temperature. 20 µL was used as the injector volume and separation was performed under isocratic elution condition using acetonitrile and water (90:10) as mobile phase, under this condition the separation lasted for 12 mins.
2.5. Statistical Data Analysis
- Samples with concentrations below the Limit of detection were assigned a value of zero for all concentration values. Field blanks and spiked blanks were used to determine any background contamination. The concentration values were corrected by the blanks data and statistical analysis were performed with Analysis Of Variance (ANOVA). The ANOVA was used for comparing the difference in the group mean concentrations of the PEs in samples for significance difference in the variance of the group mean concentrations.
3. Results and Discussion
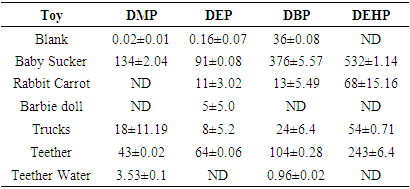
3.1. PEs in PET Drinking Water Samples
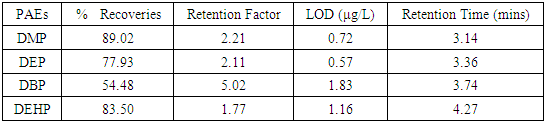
- The high values obtained for the percentage recovery studies of the PEs (57.48 - 89.00%) presented in Table 2 which compares favorably with that reported in literatures [20,22] gives credence to the efficiency of the analytical procedures adopted in the work. Values that ranged between 0.57 - 1.16μg/L obtained for the LOD demonstrated the high sensitivity of the analytical method adopted. The order of elution of the phthalates was DMP, DEP, DBP and DEH.
|
|
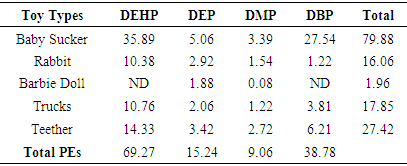
3.2. PEs in Soft Toys
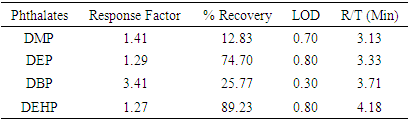
- The values obtained for the Limit of Detection (LOD), Retention Time and Response Factor and Percentage Recovery of the PEs in the soft toys are recorded in Table 4. The trend of elution of the phthalates from the column was in the order of DMP, DEP, DBP and DEHP and their retention times are shown in Table 4. Values obtained for the LOD which is between 0.30-0.80 confirmed the high sensitivity of the analytical procedures adopted.
|
|
|
4. Conclusions
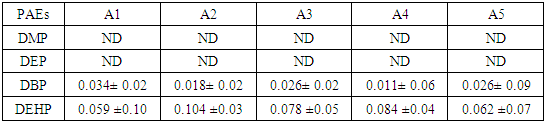
- From the results presented in this study, the following conclusions could be drawn: i. Two types of phthalates were detected in the PET bottled water samples sold in the area. DEHP was the highest detected phthalate followed by DBP, this could be based on extensive use of these two PEs industrially as well as their ubiquitous presence as environmental contaminants. ii. The concentrations of the detected phthalates were found to be significantly below the maximum levels established by FDA for bottled water (6 μg/L for DEHP in bottled water [28], iii. High significant levels of PEs in all children plastic toys samples analyzed for ages 4 months and 3 years. iv. Levels of PEs in soft toys is higher levels as compared to hard toys.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML