-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2021; 11(2): 28-36
doi:10.5923/j.chemistry.20211102.02
Received: Mar. 21, 2021; Accepted: Apr. 7, 2021; Published: Apr. 26, 2021

A Review of Biological Activities and Phytochemistry of Rhus Species
Sylvia A. Opiyo, Peter W. Njoroge, Ephantus G. Ndirangu, Kennedy M. Kuria
Department of Physical and Biological Sciences, Murang’a University, Murang’a, Kenya
Correspondence to: Sylvia A. Opiyo, Department of Physical and Biological Sciences, Murang’a University, Murang’a, Kenya.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The genus Rhus (family: Anacardiaceae, order: Sapindales) consists of more than 250 species distributed in the tropics, subtropics and temperate regions. Traditionally, extracts and products from Rhus species are regarded as important remedy and have been used extensively as part of traditional healing practices for the treatment of fungal, bacterial and protozoal infections in both humans and animals. However, scientific data to support these ethnomedicinal uses is lacking for most Rhus species. The aim of this study was to collate and review the fragmented information on ethnomedicinal, phytochemistry and biological activities Rhus species and present recommendations for future research. Peer-reviewed articles using Rhus as search term were retrieved from Scopus, Science Direct, SciFinder and Google Scholar. Various books that contained ethnopharmacological information of the plants were also consulted. In addition to anti-infective properties, Rhus extracts are also used to treat a wide range of ailments including abdominal pain, inflammation, stomach aches, fever and headaches, which may be a manifestation of infections. Most of the biological activities are attributed to flavonoids, phenolic and terpenoid compounds present in the various species. From the literature available it is evident that most of Rhus species have not been studied. Further research aimed at identification of active extracts and compounds from the plants is needed.
Keywords: Rhus species, Bioactivity, Phytochemistry, Compounds
Cite this paper: Sylvia A. Opiyo, Peter W. Njoroge, Ephantus G. Ndirangu, Kennedy M. Kuria, A Review of Biological Activities and Phytochemistry of Rhus Species, American Journal of Chemistry, Vol. 11 No. 2, 2021, pp. 28-36. doi: 10.5923/j.chemistry.20211102.02.
Article Outline
1. Introduction
- The use of plants in management of infections has been practiced for several years. Traditional medicine still plays an important role in meeting the primary healthcare needs in many developing countries [1-6]. Plants have been reported to produce secondary metabolites some of which have the capacity to combat diseases [7-11]. Despite the availability of conventional drugs, continued search for novel biologically active compounds is unavoidable since most of the available drugs have limitations in terms of side effects and drug resistance [1,12]. Currently, researchers have focused on determining efficacy of medicinal plant through in-vivo and in-vitro experiments, and isolation and characterization of bioactive compounds [13-19]. This has led to the identification of several important biologically active compounds including terpenoids, alkaloids, steroids, flavonoids and quinones [20-24]. Such compounds represent an important source of drugs in the process of developing new pharmacologically active compounds. The genus Rhus (family: Anacardiaceae, order: Sapindales) consists of more than 250 species of deciduous trees and shrubs, mainly distributed in the tropics, subtropics and temperate regions. Rhus species are widely used in modern and traditional medicine for management of various infections. However, the scientific data to support the ethnomedicinal uses is lacking for most Rhus species since only a few of the species have been subjected to scientific evaluation. The paper presents a review of Rhus species as potent medicinal plants by highlighting their traditional applications as well as the recent findings for novel pharmacological and clinical applications.
2. Ethno-Medicinal Uses of Rhus species
- Rhus species have been used for numerous applications. Rhus chinensis extracts are use treat coughs, diarrhea, dysentery, fever, jaundice, hepatitis, malaria, snake bite and rheumatism [25]; R. coriaria extracts are used to treat wounds, indigestion, anorexia, hemorrhages, hyperglycemia, stomach diseases, fever, dermatitis, diabetes, obesity, paralysis, colitis, dysentery, hemoptysis, analgesic and conjunctivitis [26-28]; R. glabra extracts are used to control diarrhoea, fevers, general debility, sore mouths, sore throats, rectal bleeding, uterine prolapse, vaginal discharge, burns and skin eruptions [29,30]; R. javanica L. extracts are used to treat dysentery, diarrhea, spermatorrhea and malaria [31]; R. trilobata extracts are used to treat gastrointestinal diseases and cancer [32]; R. tripartita is used to treat digestive diseases, wound, inflammatory conditions, gastrointestinal and cardiovascular disorders, diarrhea and ulcers [33,34]; R. tripartitum is used to treat diarrhoea, dysentery and ulcers [33]; R. succedanea L. is used to treat diarrhoea and dysentery [26]; R. typhina is used to treat abdominal pain, diarrhea, infected wounds, sore throats [35] and R. verniciflua Stokes is used to treat abdominal disorders, obesity, allergic inflammatory, allergic contact dermatitis, swelling, angiogenesis, Parkinson’s disease, Huntington’s disease, and cancer [35,37]. Given the importance of the plants in traditional medicine, further research aimed at identification of the bioactive compounds is necessary.
3. Chemical Composition
- Previous phytochemical studies have shown that Rhus species are rich in secondary metabolites such as flavonoids, urushiols, and terpenoids (Table 1). Flavonoids reported from the plants include kaempferol (1), 7-O-methyl isokaemferide (2), quercetin (3), 7,3′-O-dimethylquercetin (4), quercetin-3-O-glucoside (5), quercitrin (6), rutin (7), quercetin-3-O-α-L-(3′′-O-galloyl)-rhamnoside (8), myricetin (9), myricetin-3-O-β-glucoside (10), myricetin 3-rhamnoside (11), fisetin (12), apigenin (13), genkwanin (14), apigenin dimethyl ether (15), luteolin (16), 7-O-methyl luteolin (17), 3', 4', 7-trihydroxyflavone (18) and 5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one (19). Flavanoids reported from Rhus species include naringenin (20), 7-O-Methyl naringenin (21), eriodictyol (22), eriodictyol-7-methyl ether (23), eriodictyonone (24), 3′, 5, 5′, 7-tetrahydroxy flavanone (25), hesperetin (26), 7-O-methyl hesperetin (27), hesperetin 7-rutinoside (28), butin (29), garbanzol (30), fustin (31), taxifolin (32), catechin (33), gallocatechin (34), epicatechin (35), epicatechin-3-O-rhamnoside (36), epigallocatechin (37) and mollisacasidin (38). Isoflavonoids: daidzein (39) and orobol (40) were also reported. Chalcones from the plants include butein (41), cilicione-B (42), phloretin (43), phlorizin (44), rhusopolyphenol A (45), rhusopolyphenol B (46), peapolyphenol C (47), rhusopolyphenol D (48), rhusopolyphenol E (49), rhusopolyphenol F (50) and 3,4,2’,4’-pentahydroxydihydrochalcone (51). Biflavonoids reported form the plants are volkensiflavone (52), morelloflavone (53), rhuspartin (54), agathisflavone (55), rhusflavanone (56), robustaflavone (57), masazinoflavanone (58), hinokiflavone (59), 2′′,3′′-dihydrohinokiflavone (60), amentoflavone (61), 3′,8-binaringenin (62), 2,3-dihydroamentoflavone (63), 7,7′′-di-O-methyltetrahydroamentoflavone (64), cupressuflavone (65), mesuaferrone-A (66), mesuaferrone B (67), 2′,4′-dihydroxychalcone-(4-O-5′′′)-4′′,2′′′,4′′′-trihydroxychalcone (68) and rhuschalcone-1 (2′,4′′,2′′′-trihydroxy-4′,4′′′-dimethoxy-4-O-5′′′-bichalcone) (69). Calodenone (70), Rhuschromone (71) and 7,8,9,13-tetrahydroxy-2-(3,4-dihydroxyphenyl)-2,3-trans-3,4-cis-2,3,10-trihydrobenzopyrano[3,4-c]-2-benzopyran-1-one (72) were also reported from Rhus species. Anthocyanins such as cyanidin -3-O-glucoside (73), cyanidin-3-O-(2′′galloyl)-galactoside (74), 7-O-methyl-cyanidin-3-O-galactoside (75), 7-O-methyl-cyanidin-3-O-(2′′galloyl)-galactoside (76), delphinidin-3-O-glucoside (77), 7-O-methyl-delphinidin-3-O-(2’’ galloyl)-galactoside (78), sumadin A (79) and sumadin B (80) have been reported. Aureusidin (81) and coumarins scoporone (82), 5-formylmellein (83) and 5-hydroxymethylmellein (84) were also reported. Xanthones reported from the plants include 2,3-dihydroxy-7-methyl xanthone (85), 2,3,6-trihydroxy-7-hydroxymethylene xanthone-1-carboxylic acid (86), 2-methoxy-4-hydroxy-7-methyl-3-O-β-D-glucopyranosyl xanthone-1,8-dicarboxylic acid (87) and 2-hydroxy-7-hydroxymethylene xanthone-1,8-dicarboxylic acid 3-O-β-D-glucopyranosyl-(2’→3’’)-3’’-O-stigmast-5-ene (88).
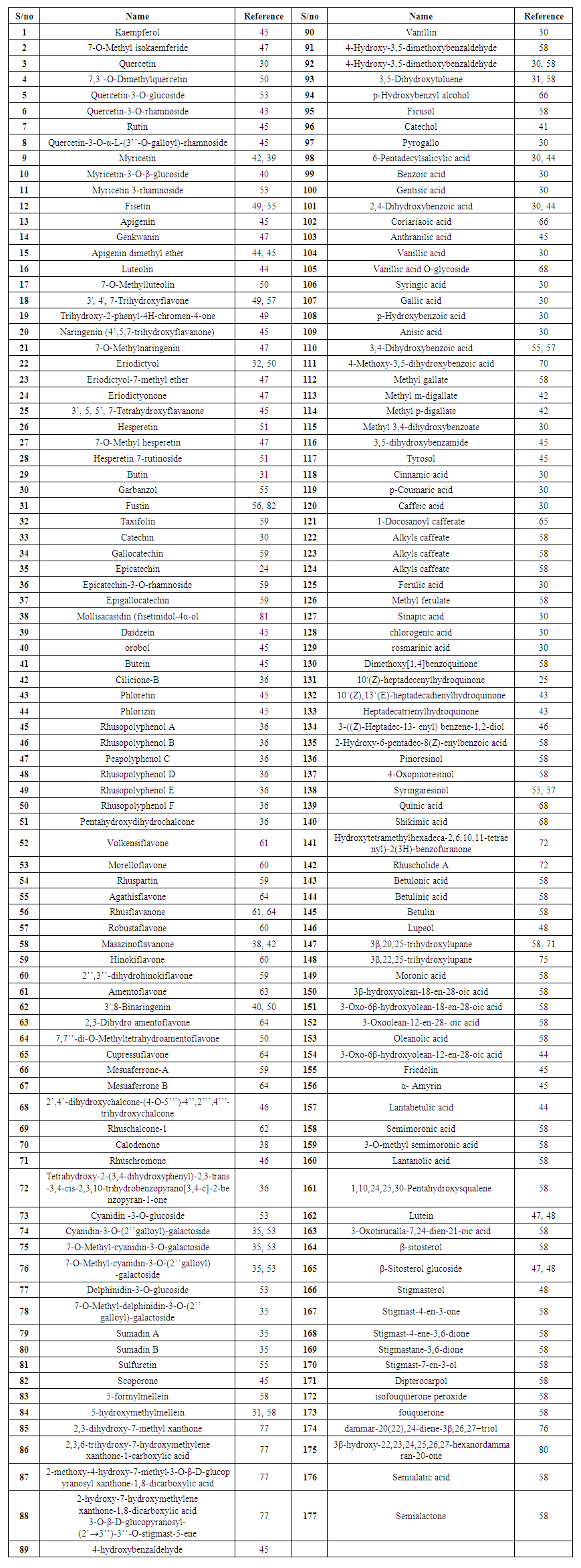 | Table 1. Compound from Rhus species |
4. Biological Activities and Bioactive Compounds
- Previous biological activity studies on Rhus species have focused majorly on the fruits because of their widespread use as a dried spice. Most of the studies have used either ethanol or water based extracts [27,47]. Biological activities reported from extracts from the plant species include antimicrobial, cytotoxicity, anticancer, antioxidant, antiviral, anti-inflammatory and antimalarial activities. However, in most cases the bioactive principles are not known as most studies end after determination of bioactivities to the extracts. Extracts from Rhus species have been reported to have antimicrobial activity against a wide range of pathogenic microbes including Aeromonas hydrophila, Aspergillus flavus, A. fumigatus, A. Niger, Bacillus cereus, B. subtilis, Candida albicans, Enterobacter aerogenes, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Listeria monocytogenes, Microsporum gypsum, Mycobacterium tuberculosis, Penicillium notatum, Propionibaterium acnes, Proteus vulgaris, Pseudomonas aeruginosa, P. syringae, Ralstonia solanacearum, Salmonella enteric, S. typhi, Shigella dysentariae, Staphylococcus aureus, S. epidermidis, S. faecalis, Streptococcus mutans, S. pyogenes, Trichophyton mentagrophytes, T. rubrum, Xanthomonas axonopodis X. oryzae and Yersinia enterocolitica [48,78]. The antimicrobial compounds reported from the plants are 2’,4’-dihydroxychalcone-(4-O-5’’’)-4’’,2’’’,4’’’-trihydroxychalcone (68), rhuschromone (71) and 3-(Z)-heptadec-13- enyl) benzene-1,2-diol (134) from R. natalensis root bark [46]; epicatechin (35), lupeol (146), β-sitosterol (164), β-sitosterol glucoside (165) and stigmasterol (166) from R. natalensis root bark [48]; 2,3-dihydroxy-7-methyl xanthone (85), 2,3,6-trihydroxy-7-hydroxymethylene xanthone-1-carboxylic acid (86), 2-methoxy-4-hydroxy-7-methyl-3-O-β-D-glucopyranosyl xanthone-1,8-dicarboxylic acid (87) and 2-hydroxy-7-hydroxymethylene xanthone-1,8-dicarboxylic acid 3-O-β-D-glucopyranosyl-(2’→3’’)-3’’-O-stigmast-5-ene (88) from R. coriaria L. seeds (Singh et al 2011); methyl gallate (112) from R. verniciflua [57]; 7-O-methylnaringenin (21) from R. retinorrhoea [50]; fisetin (12), 3', 4', 7-Trihydroxyflavone (18) and gallic (107) from R. verniciflua [79]; and 1,10,24,25,30-pentahydroxysqualene (161) and dammar-20(22),24-diene-3β,26,27−triol (174) isolated from R. taitensis [76].Cytotoxic compounds reported from Rhus species are myricetin-3-O-glucopyranoside (10), epicatechin (35), mesuaferrone-A (66), and 2′′3′′-dihydrohinokiflavone (60) from stem bark of R. tripartita [59]; and 4-(2,6-dihydroxy-4- methoxyphenyl)-4-oxobutanoic acid, trichocarpol A, trichocarpol B, trichocarpol C, trichocarpol D and trichocarpol E, from R. trichocarpa roots [80]. Anticancer compounds reported include kaempferol (1), quercetin (3), myricetin (9), methyl m-digallate (113), methyl p-digallate (114), and betulinic acid (144) from R. copallinum fruit [42]; fustin (31) and sulfuretin (81) from R. verniciflua [81] agathisflavone (55), succedaneaflavanone (58), cupressuflavone (65), mesuaferrone A (66) and from R. Parviflora fruits [64]; and cilicione-b (42) and α,3,4,2’,4’-pentahydroxydihydrochalcone (51) from R. verniciflua bark [36].Antioxidant compounds isolated from the plants are epicatechin-3-O-rhamnoside (36) and rhuspartin (54) from stem bark of R. tripartita (Alqahtani et al 2019); 7-O-methyl isokaemferide (2), rutin (7), genkwanin (14), 7-O-methyl naringenin (21), eriodictyol-7-methyl ether (23), eriodictyonone (24), 7-O-methyl hesperetin (27) and hesperidin (28) from aerial parts of R. natalensis [47]; Myricetin-3-O-β-glucoside (13), taxifolin (32) from R. tripartita [40,59]; quercetin (3) from R. verniciflua [58]; hesperetin from R. coriaria [51]; butin (29) from R. verniciflua [55]; fustin (31) from R. verniciflua [82] and sulfuretin (81) from R. verniciflua [83]. Antiviral compounds isolated from the plants include 5-hydroxy-7-(3,7,11,15-tetramethylhexadeca-2,6,10,11-tetraenyl)-2(3H)-benzofuranone (141) and 5-hydroxy-3- (propan-2-ylidene)-7-(3,7,11,15-tetramethylhexadeca- 2,6,10,11-tetraenyl)-2(3H)-benzofuranone (142), betulonic acid (143), Moronic (149), and 3-oxo-6β-hydroxyolean-18-en-28-oic acid (151) from R. chinensis [72,73]; butin (29), fisetin (12), sulfuretin (81) and methyl gallate (112) from R. verniciflua [55,84]; agathisflavone (55), robustaflavone (57) and succedaneaflavanone (58) from R. succedanea [60]; amentoflavone (61) from Rhus pyroides [63] and 2’’,3’’-dihydrohinokiflavone (60) from R. tripartita stem bark [59]. Anti-inflammatory compounds reported from Rhus species are 5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one (19) and quercetin (3) from R. mysorensis leave [49]; myricetin-3-O-β-glucoside (10), taxifolin (32) from R. tripartita [40]; fisetin (12) from R. mysorensis leaves [49] and 1-docosanoyl cafferate (112) from R. verniciflua stem bark [65]. Antimalarial reported from the plants are genkwanin (14), 7-O-methylluteolin (17), 7-O-methylnaringenin (21) and eriodictyol (22) from R. retinorrhoea leaves [31,50].
5. Conclusions
- Whereas the genus Rhus consists of more than 250 species, only a few of the plants have been subjected to phytochemical investigation. The few species that have been studied include R. alata, R. chinensis, R. copallinum, R. coriaria, R. cotinus, R. flexicaulis, R. glabra, R. javanica, R. leptodictya, R. leptodictya, R. mysorensis, R. natalensis, R. pachyrrhachis, R. parviflora, R. pyroides, R. retinorrhoea, R. succedanea, R. taitensis,, R. tripartita, R. tripartitum, R. typhina, R. verniciflua and R. virens. Given the important role of Rhus species in modern and traditional medicine, further phytochemical investigation of the unstudied species is necessary to identify bioactive compounds. It is also necessary to determine the bioactivity of the already identified compounds.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML