-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2021; 11(1): 18-21
doi:10.5923/j.chemistry.20211101.03
Received: Feb. 10, 2021; Accepted: Mar. 5, 2021; Published: Mar. 15, 2021

The Chemistry of the Weyl-Salkowski Test for Creatinine
Francisco Sánchez-Viesca, Reina Gómez
Organic Chemistry Department, Faculty of Chemistry, National Autonomous University of Mexico, Mexico City (CDMX), Mexico
Correspondence to: Francisco Sánchez-Viesca, Organic Chemistry Department, Faculty of Chemistry, National Autonomous University of Mexico, Mexico City (CDMX), Mexico.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Creatinine is an important biomarker, thus the analytical chemistry related to it is relevant. The Weyl-Salkowski assay for creatinine is based on the reaction of this imidazolidinone derivative with sodium nitroprusside in alkaline medium, followed by acidification after the initial colours observed, being Prussian blue the final product. The fine structure of the reagent, the anionic transition metal complex, adds interest to the subject since there are two theories regarding the electron distribution in the nitrosyl-metal bond. These differences explain or not the chemical deportment of the reactants. In this communication the reaction process is cleared up. The study of this test leads to unify two theories concerning the nature of the nitrosyl-metal bond, and two alternatives are given to attain this.
Keywords: Analytical chemistry, Creatinine, Reactive intermediates, Salkowski test, Sodium nitroprusside, Weyl test
Cite this paper: Francisco Sánchez-Viesca, Reina Gómez, The Chemistry of the Weyl-Salkowski Test for Creatinine, American Journal of Chemistry, Vol. 11 No. 1, 2021, pp. 18-21. doi: 10.5923/j.chemistry.20211101.03.
1. Introduction
- The Weyl-Salkowski test is based on the interaction of creatinine with sodium nitroprusside in alkaline medium, followed by acidification with acetic acid after the initial coloured steps.Creatinine is a poly-functional compound with imidazolidine-imidazoline structures. It is a cyclic guanidine compound with a lactam group, an active methylene group, an enamine, a guanyl group and a special imido like group.Its reactivity was studied, as well as the electron structure of the reagent, the transition metal-nitrosyl complex.Since the chemistry of this test is unknown, we provide it and it is discussed in detail. This communication is a follow up of our studies on the chemistry of other colour tests, [1-5].
2. Antecedents
- Creatinine, 2-imino-1-methylimidazolidin-4-one and its tautomer, 2-amino-1-methylimidazoline-4-one, is the anhydride of creatine, that is, a lactam obtained by cyclocondensation of creatine, N-methyl-N-guanylglycine, which is present in muscular tissue of many vertebrates, Figure 1.
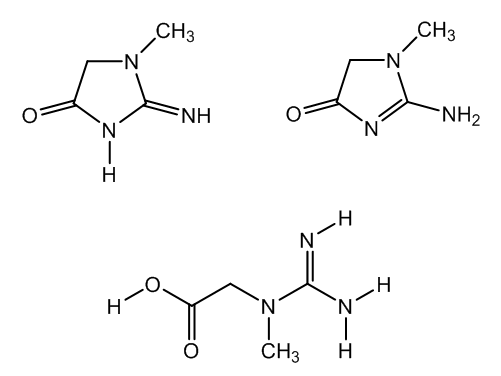 | Figure 1. Creatinine tautomrs and creatine |
3. Discussion
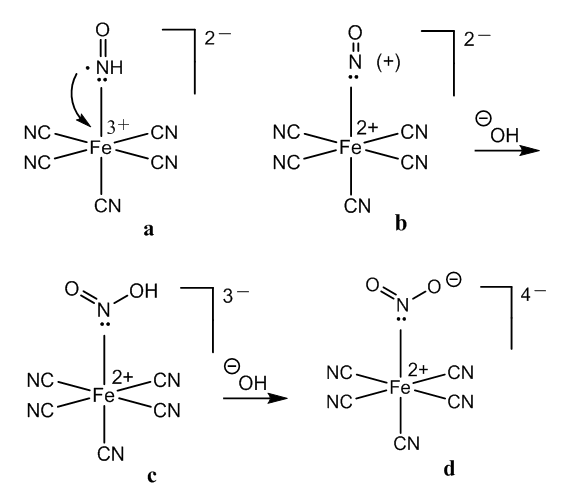
- For understanding the chemistry of the Weyl-Salkowski test for creatinine is very important clear up the electron structure of the nitroprusside anion. There are two points of view about the bond of the nitrosyl group in this metal-nitrosyl complex.The NO ligand can be considered neutral since nitric oxide is a neutral molecule, [14]. Thus, five CN- groups and one Fe3+ ion form a 2- ferrate anion, [15]. The conception of simple coordination in the nitrosyl-metal bond is in accordance with the fact that nitroprusside releases nitric oxide, a potent vasodilator effective in the lowering of blood pressure, [16].Pauling suggested that since nitric oxide is a free radical, this electron can be transferred to the iron atom, which is then Fe2+, and a positive charged nitrosyl results, [17]. This redox reaction can be explained by Kossel’s theory: the accumulated Fe3+ positive charge diminishes, the positive charge being now in two atoms, [18].Now let’s see the reaction of creatinine in alkaline medium. Creatinine has a –CH2-CO- group which can condense with aldehydes in drastic conditions, by melting the reactants in an oil bath, [19-21].However, there is in the creatinine molecule an imido like group, with an enamine instead of a carbonyl group. The central N-H in this special group is highly reactive in alkaline medium, yielding a salt. The mentioned active methylene group is vicinal to a lactam, not to a ketone, and is less reactive compared to an imide.Therefore, there are several reaction possibilities with the nitroprusside: hydroxyl group and creatinine salt reacting with Fe3+ and Fe2+ ferrates.In the model of simple coordination of nitric oxide in the nitroferrate, Figure 2, a, the creatinine anion can displace easily the neutral nitric oxide molecule. The ligand exchange reaction accounts for a salt formation, Figure 2, b.
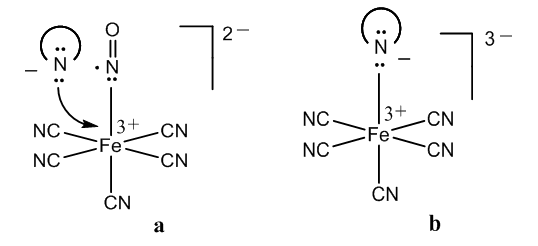 | Figure 2. Halochromism in the organometallic complex |
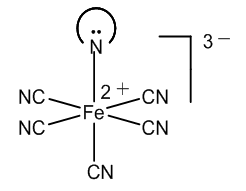 | Figure 3. Creatinine with covalent bond to iron |
 | Figure 4. Formation of [Fe(CN)5NO2]4- |
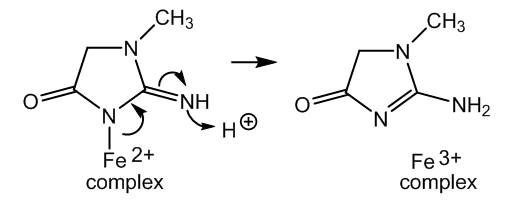 | Figure 5. Detachment of creatinine from complex in Figure 3 |
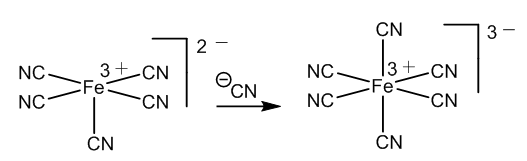 | Figure 6. Formation of hexacyanoferrate(III) |

4. Conclusions
- The chemistry of the Weyl test for creatinine can be explained better using the neutral nitrosyl structure in the nitroprusside. The red colour comes from halochromism in an organometallic complex. The change to yellow is due to formation of a covalent bond in this molecule, instead of a salt.Addition of acetic acid according to Salkowski removes the creatinine, neutralizing the anion and then forming creatininium ions. The vacant coordination site is filled by a cyanide group and Prussian blue is obtained.However, the theory of a three electron bond in the nitrosyl group is needed to explain the presence of Fe2+ ions, which in addition to Fe3+ are necessary to form Prussian blue.Therefore, two theoretical alternatives are given with the aim of unify the different interpretations about the nature and bond type of the nitrosyl ligand in sodium niroprusside.
ACKNOWLEDGEMENTS
- Thanks are given to co-worker Martha Berros for support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML