-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2020; 10(3): 45-49
doi:10.5923/j.chemistry.20201003.02
Received: Oct. 13, 2020; Accepted: Nov. 13, 2020; Published: Nov. 28, 2020

Application of Dynamic Vapour Sorption Technique to Study Dry Powder Inhalation Formulations
Sunita Sule, Sushama Ambadekar
Department of Chemistry, Institute of Science, Fort, Mumbai, India
Correspondence to: Sunita Sule, Department of Chemistry, Institute of Science, Fort, Mumbai, India.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Consistency of homogeneity and performance within batches in a dry powder inhaler (DPI) formulation can be a challenge due to the effect of physico-chemical properties of the drugs and excipients, manufacturing process and environmental condition. Spray drying techniques can be evaluated as an alternative to the historic ordered mix / physical blending techniques to reduce the some of these effects. Dynamic vapor sorption experiments were conducted to study the effect of the manufacturing process on water uptake property of DPI formulation in presence of increasing humidity. Two DPI formulations were evaluated, a conventional ternary blend and a spray dried formulation of Ciprofloxacin Hydrochloride an antibiotic, Salbutamol Sulphate a bronchodialator and Mannitol a mucolytic agent. Although both the formulations did not exhibit significant water uptake after the absorption-desorption cycle, an increase in water uptake of ~ 1.5% and ~ 3.5% was observed for the spray dried formulation at humidity above 80% and 90% respectively. This could be partially attributed to the porous structure of the spray dried formulation. The vapour sorption data offers insights that the spray dried material is sensitive to water uptake at higher humidity, this information can be used towards designing appropriate packaging material and defining environmental conditions during manufacturing, handling and storage of the spray dried formulations.
Keywords: Ternary blend, Spray drying, Dynamic vapour sorption
Cite this paper: Sunita Sule, Sushama Ambadekar, Application of Dynamic Vapour Sorption Technique to Study Dry Powder Inhalation Formulations, American Journal of Chemistry, Vol. 10 No. 3, 2020, pp. 45-49. doi: 10.5923/j.chemistry.20201003.02.
Article Outline
1. Introduction
- Earliest use of inhalation route for drug delivery can be traced back to smoking of datura preparations almost 4000 years ago in India [1]. However, Dry powder inhalers (DPI) were commercially available from the mid 90’s with the launch of Aerohalor of Abbott [1]. Since then there has been considerable improvements in optimization of drugs, excipients, manufacturing process and dry powder inhaler devices. i.e. improving the efficiency of the dry powder inhaler devices. In addition to the improvements in the DPI product there has been a continous improvement in the analytical techniques used for the characterization and release testing of inhalation products. One such technique; Dynamic vapor sorption is studied in this research article.In general pharmaceutical formulations for dry powder inhalers are prepared as a homogeneous physical mixture or blend of excipients and drug particles [2]. Some of the factors that affect the bulk and flow properties of these blends are particle size, shape, morphology, surface roughness, density, hygroscopicity and age of the drug and excipients as well as the forces such as van der Waal forces, capillary forces and electrostatic forces to which they are exposed during their processing cycle [4,5,7]. Variability in these factors lead to inter batch variabilities affecting handling, manufacturing and the performance of the product. Spray drying technique can be used commercially to manufacture formulation with consistent particle size with reduced interbatch variability [6]. Powder formulations either a blended physical mixtures or a spray dried; need to be studied for a multiple of charcteristics during development to ensure consistent quality in the product. Powder characterization can be broadly classified as thermal characterization and Physico-chemical characterization. Thermal characterization techniques like Differntial Scanning Calorimetry and Thermogravimetric analysis provide insights to characteristics like Polymorphism, crystallinity and thermal stability. Hygroscopicity is a physico chemical property describing a material ability to absorb / adsorb moisture from the environment [7]. Hygroscopicity can adversely affect the stability, performance and powder flow of a formulation blend. Insight on hygroscopicity of a formulation contributes in establishing its processing conditions, packaging requirements and storage condition. The European pharmacopoeia [8] describes a simple procedure for measuring hygroscopiocity of a substance, consisting of exposing sample at 80% humidity for 24 hrs at 25 deg C and calculating the % increase in mass by weight. In this study Dynamic Vapour sorption (DVS) was used to study hygroscopicity of a formulation manufactured by different process. The DVS instrument measures the uptake and loss of water (or Solvent) from a pre dried sample which is exposed to contolled humidity in multiple steps from 0-95% at a constant temperature [9].
2. Material and Methods
- 2.1. The raw materials Salbutamol sulphate a bronchodilator (Cipla Ltd), Ciprofloxacin Hydrochloride an antibiotic (Aarti Industries) and Mannitol a mucolytic agent (Merck) were used to prepare test formulations. 2.2. Preparation of Test Formulations Two formulations were prepared, a ternary blend and a spray dried formulation.Ternary blend containing three drugs Salbutamol Sulphate, Ciprofloxacin Hydrochloride and Mannitol in the ratio ~ 0.24: 36.1: 80 was prepared by sieving through size 80 mesh to remove any agglomerates and tumble blended in a stainless steel jar for 20 mins. The blend was allowed to equilibrate for minimum 24 hrs before commencing testing. The spray drying was done using LabUltima Spray dryer, India. The co-spray dried particles were prepared by spraying a 2% w/v aqueous solution containing Salbutamol Sulphate: Ciprofloxacin Hydrochloride: Mannitol in the ratio ~ 0.24: 36.1: 80 using a 0.7 mm two fluid spray nozzle. The feed rate was set at 2 ml/min, the atomizing pressure between 1.3-1.5 bar and aspirator at 55 m3/hr. The Inlet temperature was set between 100-130°C to obtain an outlet temperature in the range of 90-95°C. 2.3. Scanning Electron MicroscopyMorphology of both the formulations was determined using scanning electron microscope (Model Supra 5; Carl Zeiss, Germany) at 10 kV. The samples were mounted on a metal stub with double-sided adhesive tape and were gold coated under a vacuum prior to imaging. 2.4. Dynamic Vapour SorptionWater sorption isotherms were measured gravimetrically using Dynamic Vapour Sorption, DVS Resolution (Surface Measurement Systems, UK) at 25°C. The experiments were performed at atmospheric pressure, using 200 mL/min of dry air as the carrier gas. Water vapour concentrations were measured and maintained with 10% steps between concentrations from 0% to 90% RH followed by 5% step to 95% RH. The relative humidity was then decreased in a similar manner. Samples weighing between 10 and 20 mg were dried at the experimental temperature for 10 h under dry airflow before introducing different concentrations of water vapour. Both formulations; the ternary blend and a spray dried formulation of Ciprofloxacin HCl, Salbutamol Sulphate and Mannitol were equilibrated for atleast 48 hours before testing using the Dynamic Vapour Sorption instrument as described above. The instrument measures the uptake and loss of moisture during this cycle gravimetrically using a high resolution micro balance (± 0.1 μg). The rate at which the material equilibrates at each humidity level as well as the overall shape of the adsorption / desorption curve was studied for the two formulations.
3. Results and Discussion
- From the SEM it can be seen that the finer Salbutamol Sulphate and Ciprofloxacin HCl particles are adhered to the irregular shaped slighlty larger mannitol particles (Fig 1). The appearance of the spray dried particles (Fig 2) is porous and spherical in shape inline with the spray drying process.
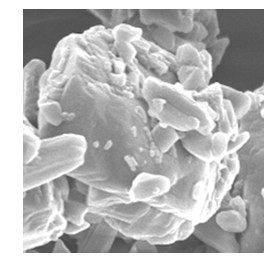 | Figure 1. SEM - ternary blend |
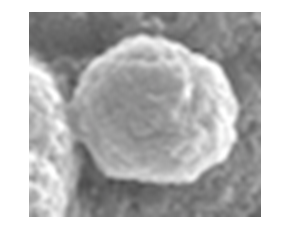 | Figure 2. SEM - Spray dried Blend |
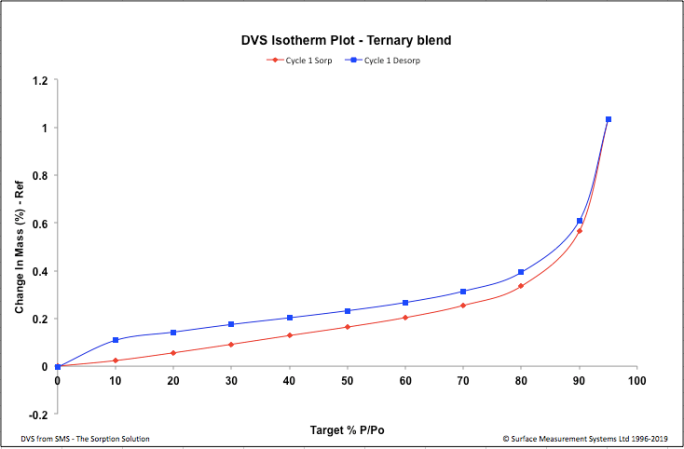 | Figure 3. Ternary Blend – DVS Isotherm Plot |
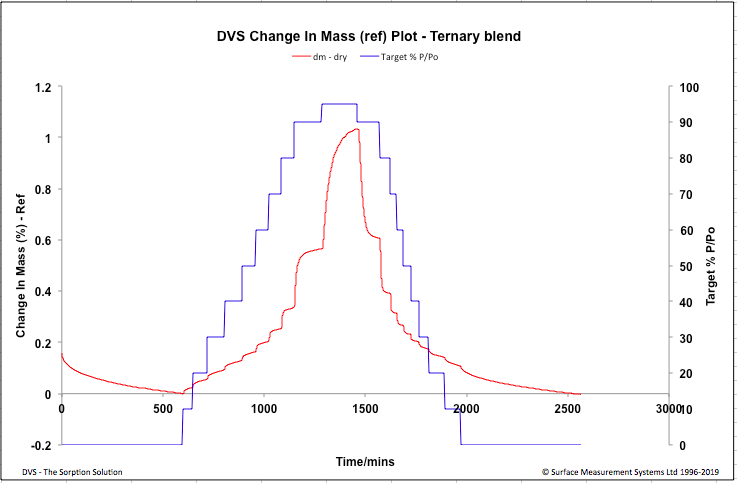 | Figure 4. Ternary Blend - Change in Mass Plot |
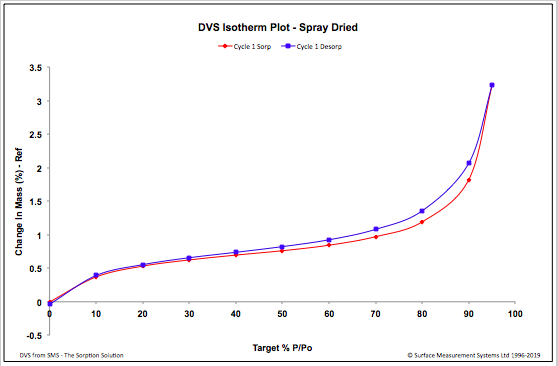 | Figure 5. Spray Dried – DVS Isotherm Plot |
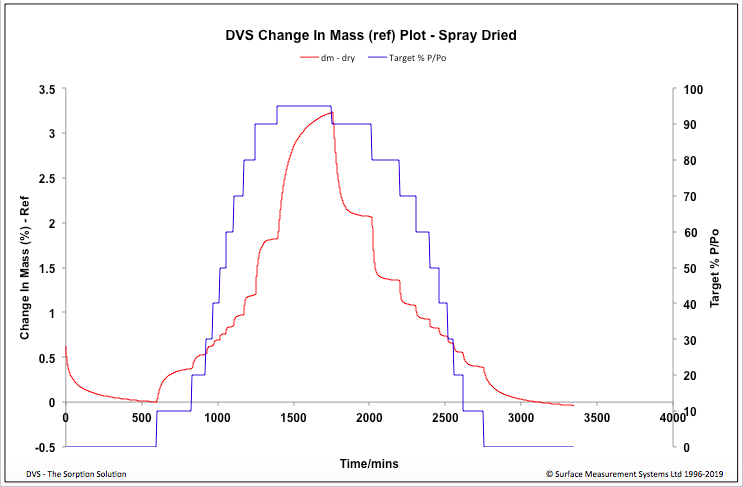 | Figure 6. Spray dried - Change in Mass Plot |
4. Conclusions
- In this study the DVS methodlogy was used to study the water uptake behaviour of a formulation manufactured by two different techniques, in presence of variable humidity conditions. Although both the formulations did not exhibit significant water uptake after the desorption cycle, an increase in water uptake was observed for the spray dried formulation at high humidity above 80%. The spray drying process has lead the formulation to become slightly hygroscopic, this information would help in taking informed decisions when optimizing manufacturing and handling process, developing packaging material and storage conditions of the spray dried formulation.
ACKNOWLEDGEMENTS
- The authors would like to thank Surface Measurement System for the access and support of Dr Naderi, Dr Meishan, Dr Bendre with the DVS studies.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML