-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2020; 10(3): 39-44
doi:10.5923/j.chemistry.20201003.01
Received: Oct. 14, 2020; Accepted: Nov. 2, 2020; Published: Nov. 15, 2020

Insecticidal Activity of Elaeodendron schweinfurthianum Extracts and Compounds against Sitophilus zeamais Motschulsky
Sylvia A. Opiyo
Department of Physical and Biological Sciences, Murang’a University, Murang’a, Kenya
Correspondence to: Sylvia A. Opiyo, Department of Physical and Biological Sciences, Murang’a University, Murang’a, Kenya.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Agricultural production is constrained by pests which cause serious post-harvest losses in African. Maize weevil (Sitophilus zeamais Motschulsky) is one of the most destructive insect pests of maize. Effective synthetic insecticides are commercially available but many have serious shortcomings including accumulation in the food chain and insect resistance, hence the need to search for novel insecticidal agents. Plants provide an alternative source of drugs that are safer to both humans and environment. Elaeodendron schweinfurthianum (Loes) is used traditionally to manage bacterial and fungal infections such as wounds, primary symptoms of syphilis and diarrhea. This study aimed at determining the efficacy of extracts and some compounds isolated from Elaeodendron schweinfurthianum in controlling maize weevil in stored maize grain. n-Hexane, ethyl acetate and methanol extracts from the stem bark of the plant exhibited repellent, mortality and adult emergence inhibition activities against maize weevil. The most potent compounds were 3-oxofriedelane, 3-oxo-29-hydroxyfriedelane, 3-oxofriedelan-28-al and α-amyrin acetate. The findings from this study show that extractes from Elaeodendron schweinfurthianum are effective in controlling maize weevil.
Keywords: Elaeodendron schweinfurthianum, Maize weevil, Repellence, Mortality, Growth inhibition
Cite this paper: Sylvia A. Opiyo, Insecticidal Activity of Elaeodendron schweinfurthianum Extracts and Compounds against Sitophilus zeamais Motschulsky, American Journal of Chemistry, Vol. 10 No. 3, 2020, pp. 39-44. doi: 10.5923/j.chemistry.20201003.01.
Article Outline
1. Introduction
- In Africa, crop production is constrained by field and storage pests, which cause serious post-harvest losses [1,2]. Maize weevil (Sitophilus zeamais Motschulsky.) is one of the most destructive insect pests of maize. Several methods are used in in attempt to control damages caused by insect in stored grains including smoking, sun-drying, heating and use of synthetic chemical [3,4]. The successful wide scale use of synthetic insecticides commencing with the organochlorines in the middle 1940s followed by the later use of organophosphates, carbamates, pyrethroids and avermectins has been reported [3,4]. Some of the synthetic pesticides have shortcomings including side effects to non-target organisms leading to them being banned from use [5-8]. Additionally, the high cost of synthetic insecticides limits their accessibility to peasant farmers [9]. Plants have been proved to be an alternative source of readily available extractives that are safer to both humans and environment [10-19].Plants belonging to the genus Elaeodendron are characterized by the presence of steroids, flavonoidsand and terpenoids [20-22]. Biological activities of Elaeodendron species include insect feeding deterrent, antifungal, antibacterial, cytotoxic and antiviral [20-22]. Elaeodendron schweinfurthianum (Loes) which is widely distributed in tropical Africa is used traditionally to treat bacterial and fungal infections including wounds, primary symptoms of syphilis and diarrhea [23]. This paper reports the efficacy of extracts and compounds isolated from Elaeodendron schweinfurthianum in controlling S. zeamais infestation in stored maize grains.
2. Materials and Methods
2.1. Plant Materials
- Elaeodendron schweinfurthianum stem bark was collected from Shimba Hills in Kenya (latitude 4° 15' 53.84'' S and longitude 39° 22' 19.61'' E). Sample identified was done at the Kenya National Museum herbarium where the voucher specimen (2008/09/04/SAO/CHEMMK) was deposited. The plant materials were chopped into small pieces, air dried and ground into fine powder using a mill.
2.2. Extraction and Isolation of Compounds
- Powdered plant material (2 kg) was extracted sequentially with n-hexane, EtOAc and MeOH by soaking the material in the solvent for seven days. The mixture was filtered and solvent evaporated at reduced pressure to yield 15 g, 100 g and 210 g of n-hexane, EtOAc and MeOH extracts, respectively. n-Hexane extract (10 g) was chromatographed over silica gel-packed column (2.5 x 60 cm, 150 g) and eluted with n-hexane - ethyl acetate mixture to yield 100 fractions each of 20 ml. Fractions showing similar TLC profiles were combined resulting into three pools (I-III). Pool I (1 g) did not show any major spot on TLC and was discarded. Pool II (3 g) crystallized out to give a white compound which on further purification using n-hexane-ethyl acetate (9:1) gave α-amyrin acetate (6) 56 mg. The mother liquor of this pool was subjected to further column chromatography with n-hexane- EtOAc (9:1) to afford stigmasterol (8) 78 mg. Pool III (3 g) also crystallized out and after re-crystallization (n-hexane-EtOAc, 9:1) afforded further stigmasterol (8) 45 mg. Ethyl acetate extracts (75 g) was chromatographed over silica gel-packed column (5 x 60 cm, 200 g) eluting with n-hexane-ethyl acetate (10% increment of ethyl acetate), ethyl acetate neat and finally with CH2Cl2-MeOH (with 10% and 20% increment of MeOH) to yield 251 fractions (20 ml each). Fractions showing similar TLC profiles were combined resulting in five pools (I-V). Pool I (8 g) on subjection to further column chromatography eluting with n-hexane-ethyl acetate (95:5, 9:1) gave α-amyrin acetate (6) 30 mg. Pool II (15 g) on further fractionation with n-hexane: ethyl acetate mixture (95:5, 9:1, 4:1) afforded α-amyrin acetate (6) 72 mg, 3-oxofriedelane (1) 65 mg and β-sitosterol (7) 54 mg. Pool III (17g) yielded stigmasterol (8) 78 mg, 3-oxofriedelan-28-al (4) 80 mg and 3α-hydroxyfriedelane (2) 83 mg. Pool IV (13 g) afforded α-amyrin (5) 77 mg and 3-oxo-29-hydroxyfriedelane (3) 93 mg on further fractionation with n-hexane: ethyl acetate mixture (4:1, 7:3). Pool V (9 g) gave lanosterol (9) 74 mg on further column chromatography eluting with n-hexane: ethyl acetate (7:3, 3:2).
2.3. Mass Rearing of S. Zeamais
- Adult weevils (S. zeamais) were obtained from infested maize grains purchased from local market and from this stock, new generation was reared on dry pest susceptible maize grains [24]. Two hundred maize weevils of mixed sexes were introduced into a two liter glass jars containing 400 g weevil susceptible maize grains [25]. The mouths of the jars were then covered with nylon mesh held in place with rubber bands and the jars left undisturbed for 35 days for oviposition. Thereafter, all adults were removed through sieving and each jar was left undisturbed for another 35 days. Emerging adult insects were collected and kept in separate jars according to their age. Adults that emerged on same day were considered of the same age [26].
2.4. Repellency Test
- The test was done according to Mwangangi and Mutisya [24] with some modifications. Transparent plastic tubings, 13 cm long x 1.3 cm diameter were used as test cylinders. Each test cylinder was plugged at one end with cotton ball containing crude extracts and compound isolated from the stem bark of E. schweinfurthianum while the other end was plugged with clean cotton ball which served as control. Actellic dust was used as a positive control. Ten-three-day old unsexed test insects were introduced at the middle of each test cylinder through a hole at the middle portion of the cylinder (0.0 cm) and let to move in any direction of their choice with scoring of distance moved measured in cm using a ruler. The score time was 24 hours after exposure and all tests were done in triplicates.
2.5. Adult Mortality Test
- Contact toxicity assay was done according to [27] with some modifications. Toxicity of the crude extracts and isolated compounds were tested against adult weevils. The test samples were mixed with talc thoroughly and the dust was admixed with 20 g of maize held in 12 cm high x 6.5 cm diameter glass jars covered with ventilated lids. To ensure a thorough admixture, the grain was put in 12 cm high x 6.5 cm diameter glass jars, dust applied and top lid replaced. The grain was then swirled within the jar until a proper admixture was realized [28]. Twenty-three-day old unsexed insect pairs were then introduced into each dish and exposed to treatments. Actellic dust was used as a positive control and all tests were done in three replicates. Maize weevils were considered dead when probed with sharp objects and there were no responses [27]. The number of dead insects in each vial was counted after 21 days after treatment to estimate maize weevil mortality as follows:
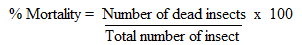 Data on percentage adult weevil mortality were corrected using Abbott’s formula [29]: PT = (Po – Pc) / (100 - Pc); Where PT = Corrected mortality (%); Po = Observed mortality (%); PC = Control mortality (%).
Data on percentage adult weevil mortality were corrected using Abbott’s formula [29]: PT = (Po – Pc) / (100 - Pc); Where PT = Corrected mortality (%); Po = Observed mortality (%); PC = Control mortality (%).2.6. Growth Inhibition Assay
- The test was done according to Ileke and Oni [27] with some modifications. 20 g of clean undamaged and uninfected corn grains were placed in 12 cm high x 6.5 cm diameter glass jars glass jars. Test materials (crude extracts and isolated compounds) were thoroughly mixed with the grains in each jar. Crude extracts and pure compounds were mixed with talc thoroughly before being applied to the grains [28]. A mixture of twenty-seven-day old unsexed maize weevils was introduced in each jar and covered with filter paper [26]. The female adults were allowed to oviposit on the seeds for 4 days. On day 5, all insects were removed from each container and the seeds returned to their respective containers. Progeny emergence (F1) was recorded at six weeks (42 days). The containers were sieved out and newly emerged adult weevils were counted [27]. At week six, the grains were reweighed and the percentage loss in weight was determined as follow:
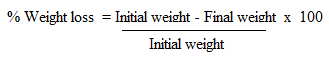
3. Results and Discussion
3.1. Phytochemical Studies
- Chromatographic fractionation of n-hexane and ethyl acetate extracts from E. schweinfurthianum stem bark afforded nine compounds (Figure 1) namely 3-oxofriedelane (1), 3α-hydroxyfriedelane (2), 3-oxo-29-hydroxyfriedelane (3), 3-oxofriedelan-28-al (4), α-amyrin (5), α-amyrin acetate (6), β-sitosterol (7), stigmasterol (8) and lanosterol (9). Structure elucidation of the compounds was earlier reported [21,30].
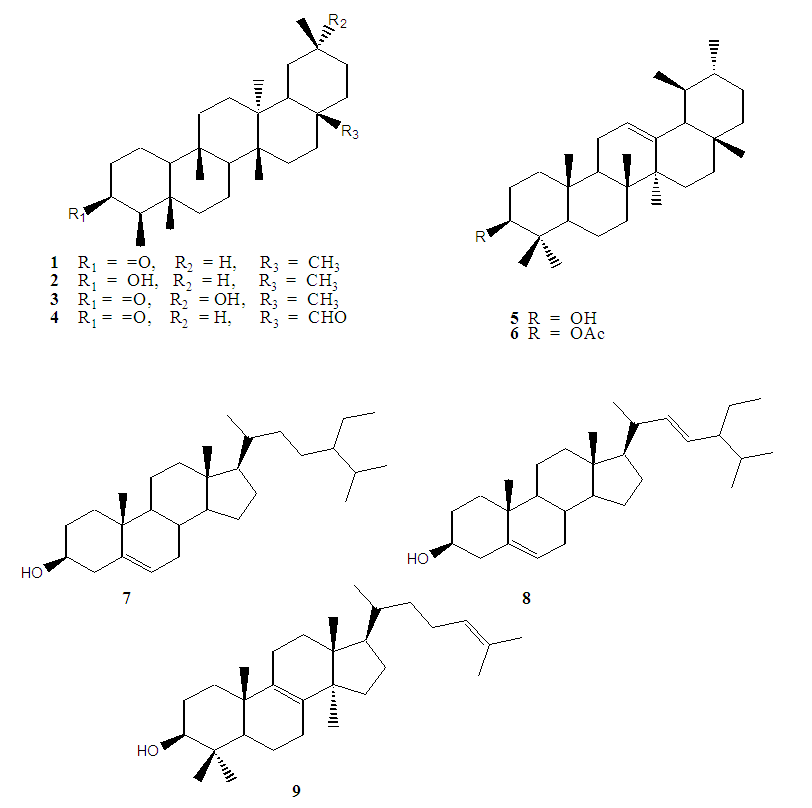 | Figure 1. Compounds isolated from stem bark of E. schweinfurthianum |
3.2. Repellent Activity
- Repellence activity of crude extracts and compounds isolated from E. schweinfurthianum against maize weevil was recorded after 24 hours of exposure and the results were as presented in Table 1. All the crude exhibited repellence activity with n-hexane extracts being the most active (mean repellency = 4.61cm) followed by ethyl acetate extract (mean repellency = 3.66 cm). All the isolated compounds also caused some repulsion against the weevils. However, all the compounds were less repellent compared to n-hexane and ethyl acetate extracts. 3-Oxo-29-hydroxyfriedelane (3) and 3-Oxofriedelane (1) were the most repellent compounds with the weevils moving 3.45 and 3.21 cm respectively away from the center of the tube. All the tested materials exhibited lower repellence activity over the test period than actellic powder which was used as a positive control.
|
3.3. Mortality Activity of Extracts and Compounds Against Maize Weevil
- The mortality effects of crude extracts and isolated compounds from E. schweinfurthianum against S. zeamais were as presented in Table 1. All the tested materials reduce the longevity of adults S. zeamais on treated maize grains. The toxicity levels of the crude extracts ranged from 43.43 to 73.2% with ethyl acetate extract exhibiting the highest toxicity followed by n-hexane extract. All the nine compounds isolated from n-hexane and ethyl acetate extracts showed varied levels of toxicity against the weevils. 3-Oxo-29-hydroxyfriedelane (3) and 3-Oxofriedelane were the most toxic to the weevils (70.0 and 58.4% respectively) 21 days after treatment compared to the other isolated compounds. However, all the tested compounds and extracts were less toxic to the weevil than actellic powder.
3.4. Growth Inhibition Activity and Weight Loss in Maize Grains
- The extracts and isolated compounds significantly reduced the progeny of S. zeamais (Table 1). For the crude extracts, methanol extract exhibited the highest growth inhibition activity followed by ethyl acetate and n-hexane extracts with percent adult emergence of 9.3 11.9 and 14% respectively. For the pure compound, 3-oxo-29-hydroxyfriedelane (3), α-amyrin acetate (6), 3-oxofriedelane (1) and 3-oxofriedelan-28-al (4) inhibited the adult emergence the most with emergence inhibitions of 11.5, 12.6, 17.6 and 19.3% respectively. Methanol extract was the best protectant of the maize grains against the weevils followed by n-hexane and ethyl acetate extracts with weight losses of 5.4, 6.2 and 9.8% respectively. The weight losses recorded in maize grains treated with the isolated compound ranged between 10.1 to 33.6% with 3-oxo-29-hydroxyfriedelane (3) and α-amyrin acetate (6) being the best grain protectants.The repellent, mortality and adult emergence inhibition tests have shown that all the extracts (n-hexane, ethyl acetate and methanol) are active against S. zeamais which is the most destructive insect pest of maize in storage. The findings were in agreement with previous studies [31-33] which reported the pesticidal activity of extracts from various plants. Extracts and compounds from Ocimum Kilimandscharicum [31], Annona mucosa [32] and Warburgia ugandensis [33] exhibited repellence, growth inhibition and insecticidal activities against Sitophilus zeamais. The isolated compounds namely 3-oxofriedelane (1), 3-oxo-29-hydroxyfriedelane (3), 3-oxofriedelan-28-al (4) and α-amyrin acetate (6) were the most potent for maize weevil control. 3-oxofriedelane (1) exhibited insecticidal and cytotoxic activities against Musca domestica and Aedes albopictus [34]. The compound also exhibited antifeedant, larvicidal and pupicidal activities against Helicoverpa armigera and Spodoptera litura [35]. Alpha-amyrin acetate (6) exhibited larvicidal and insect growth regulator effect against Anopheles Stephensi [36,37]. Compound 1, 3, 4 and 6 have been reported to exhibit antimicrobial activities [21].The use of plant extracts as to control pests is environmentally safe compared to the synthetic chemicals. From the results, the isolated compounds were less active compared to the crude extracts in the repellence, adult mortality growth inhibition tests suggesting possible synergistic effect in the extracts. More research to evaluate the synergism effects of the isolated compounds is necessary to find out which combinations might give the best activities. It is also necessary to test the efficacy of the plant in controlling other insect pests of maize.
ACKNOWLEDGEMENTS
- The authors are grateful to the National Commission for Science, Technology and Innovation (NACOSTI) and Biosciences Eastern and Central Africa Network (BecANet) for financial support. We also acknowledge the assistance from Chemistry Department, Maseno University.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML