Sushama Raju Ambadekar, Deepak Baburao Nikam
Department of Chemistry, Institute of Science, Fort, Mumbai, India
Correspondence to: Deepak Baburao Nikam, Department of Chemistry, Institute of Science, Fort, Mumbai, India.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Formaldehyde is a known carcinogen, hazardous for health. Formaldehyde is used in some of the food products as preservative to arrest microbial growth. A simple, precise, accurate, and sensitive Evaporative light scattering detection (ELSD) based High Performance Liquid Chromatography (HPLC) method is developed to analyze the selected baby foods samples. The chromatographic separation of formaldehyde was achieved with C18, 250 x 4.6 mm, 5 µm column. Mobile phase combination of 0.1% v/v formic acid in water and 0.1% v/v formic acid in acetonitrile is delivered in gradient mode for 15 min. run time at a flow rate of 1.0 mL/min. Formaldehyde lacks intrinsic chromophore, volatile in nature the sample was derivatized with 2,4-dinitrophenylhydrazine to form 2,4-dinitrophenylhydrazone, followed by ELSD detection. Method validation performed in accordance (ICH) Q2 (R1) guideline. A calibration curve plotted from 0.5 ppm to 5.0 ppm (r > 0.9954). %RSD for intra-day and inter day precision was < 5.0%. Limit of quantification (LOQ) for method was 0.5 ppm. Analysis of selected samples performed with validated method. Observed results are ranging from 0.6 ppm to 13.1 ppm.
Keywords:
Formaldehyde, Carcinogen, HPLC, ELSD
Cite this paper: Sushama Raju Ambadekar, Deepak Baburao Nikam, Formaldehyde in Baby Foods by HPLC-ELSD, American Journal of Chemistry, Vol. 10 No. 2, 2020, pp. 19-25. doi: 10.5923/j.chemistry.20201002.01.
1. Introduction
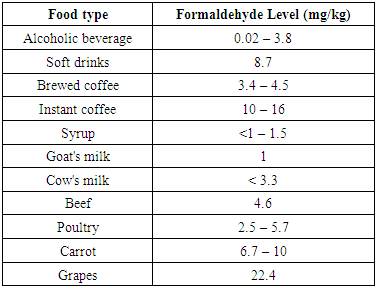
Formaldehyde (CH2O) is also known as methanal, methylene oxide, oxymethylene, methylaldehyde, oxomethane, and formic aldehyde. Its Chemical Abstracts Service (CAS) registry number is 50-00-0. Pure formaldehyde is not available commercially but is sold as 30–50% (by weight) aqueous solutions. Formalin (37% CH2O) is the most common solution. Formaldehyde is known carcinogen and hazardous for health. Occupational Health and Safety Administration (OSHA) has stated “Formaldehyde has the potential to cause cancer in humans when exposure is more than acceptable level” [1]. The TDI is the estimated amount of a substance that can be ingested daily (on body weight basis) over a lifetime without appreciable risk. Formaldehyde is found naturally at low levels in a wide range of foods such as fruits, vegetables, mushrooms and seafood. It is also a normal product of human metabolism. Ingestion of a small amount of formaldehyde is unlikely to cause any acute effect. Acute toxicity after ingestion of large amount can cause severe abdominal pain, vomiting, coma, renal injury and possible death [2]. The World Health Organization (WHO) has established a Tolerable Daily Intake (TDI) of 0.15 mg/kg body weight for formaldehyde in drinking water [3]. Centre for food safety, the Government of Hong Kong has studied Formaldehyde levels observed in natural food. Findings of study are reported in Table 1.Table 1. Levels of formaldehyde in natural food [4]
 |
| |
|
Presence of excess formaldehyde in any food product can result in adverse health effects. Hence, it is extremely important that quality and safety for all the food products is assured [5,6]. The dietary exposure per day shall be limited below 0.15 mg/kg body weight, tolerance limit set by WHO.
2. Materials and Method
All chemicals and solvents used in this study were of analytical / HPLC grade. A HPLC (Agilent Technologies, 1260 Infinity), Acetonitrile HPLC grade, Millipore Water, 2,4- Dinitrophenyl hydrazine, Formic acid HPLC grade, Formaldehyde AR grade, selected food products available in market are used as test samples, these samples are purchased from the local market. Chromatographic conditionsHPLC: Agilent Technologies, 1260 Infinity Column: Inertsil ODS 3 C18, 250 mm x 4.6 mm, 5 µ Flow: 1.0 mL/min.Injection volume: 100 µLColumn temperature: 40°C Diluent: 2, 4-DNPH solution: Acetonitrile (3:2) Run time: 15 min.Mobile phase: Water (A): Acetonitrile (B) in gradient modeGradient Program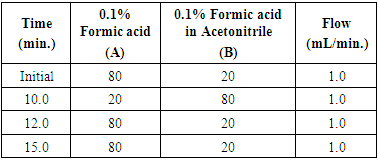 Detector ParametersDetection: Agilent 1260 Infinity Evaporative Light Scattering DetectorEvaporator temperature: 60°C Nebulizer temperature: 50°C Gas Flow rate: 1.60 SLMData rate: 80 HzLED Intensity: 100%PMT gain: 10.0Preparation of 2, 4-DNPH Solution833 mg of 2, 4-DNPH was weighed and transferred in 200 mL volumetric flask. 170 mL of Acetonitrile added to the same flask followed by 28 mL Carbon tetrachloride and 2 mL ortho- Phosphoric acid. This solution was shaken well to dissolve the reagent. This solution was transferred to 500 mL separating funnel and 200 mL water was added. Extraction was done by shaking well. The aqueous layer was separated. This solution was used for preparation of diluent.Diluent2, 4-DNPH solution: Acetonitrile (3:2).Preparation of Blank10 mL diluent and 6 mL water was taken into 20 mL volumetric flask. This flask was kept for mechanical stirring for 30 min. Volume made upto the mark with water and kept aside for 1 hr. standing.Standard Stock Solution205 mg of formaldehyde, 37% (Formalin) was weighed in 250 mL volumetric flask. Volume made upto 250 mL with water. 10 mL of this solution diluted to 100 mL with water. Transferred 1 mL of resultant solution to 100 mL volumetric flask and diluted up to the mark with water. Further, 1.0 mL of above solution is diluted to 100 mL with water.Preparation of Formaldehyde Standard SolutionIn 50 mL volumetric flask, 18 mL diluent and 2 mL of Standard stock solution of formaldehyde solution was taken. This flask was kept for mechanical stirring for 30 min. Volume made upto the mark with water and kept for 1 hr. standing (concentration of formaldehyde approx. 0.03 ppm).Preparation of Sample solution200 mg of crushed sample weighed and transferred in 50 mL volumetric flask. 18 mL of diluent and 2 mL of water was added to the flask. This flask was kept for mechanical stirring for 30 min. Volume made upto the mark with water and kept aside for 1 hr. standing.Note: Sample preparation can be adjusted to obtained the area of sample solution within range of calibration curve.Derivatization reaction used is 2,4-dinitrophenylhydrazine2,4-Dinitrophenylhydrazine used to detect the carbonyl functionality of formaldehyde. Presence of formaldehyde is indicated by a yellow or red precipitate (known as a dinitrophenyl hydrazone). Thus, 2, 4-DNP was used as a diluent for sample preparation. Note: Store Standard stock solutions, Standard solution and Sample solution at 8°C, immediately after preparation.Method Development and Method ValidationThe most widely used methods for the detection of formaldehyde are based on spectrophotometry, but other methods, such as colorimetry, fluorimetry, high-performance liquid chromatography, polarography, gas chromatography are also used. Organic and inorganic components in food products, other aldehydes and amines, can interfere with these methods of detection. ELSD is preferred for development of method for food products as it is considered as a universal detector due to the specificity of the detection method, which is based on the scattering of laser light on the non-volatile analyte particles. ELSD detector has advantage over other detectors for food samples analysis as other components, colors, additives, preservatives do not interfere in quantification, which is not possible with Gas chromatography detection and UV spectrophotometric detection [7]. Different HPLC columns with Octadecylsilane stationary phase were evaluated. However, C18, 250 x 4.6 mm, 5 µm column from Inertsil brand was found suitable. Similarly, different mobile phase compositions were tried but satisfactory separation and symmetrical peak was obtained by using gradient elution with selected composition of diluted formic acid by using gradient mode [8]. Formaldehyde do not have chromophore; quantification is done with derivatization technique. 2,4- dinitrophenyl hydrazine is used as derivatization reagent. Formaldehyde forms a hydrazone derivative upon reaction with 2,4-dinitrophenylhydrazine [9]. Use of derivatization technique enabled enhanced response for formaldehyde to ensure trace level detection. The reaction between 2, 4- Dinitrophenyl hydrazine and formaldehyde is shown in figure 1.
Detector ParametersDetection: Agilent 1260 Infinity Evaporative Light Scattering DetectorEvaporator temperature: 60°C Nebulizer temperature: 50°C Gas Flow rate: 1.60 SLMData rate: 80 HzLED Intensity: 100%PMT gain: 10.0Preparation of 2, 4-DNPH Solution833 mg of 2, 4-DNPH was weighed and transferred in 200 mL volumetric flask. 170 mL of Acetonitrile added to the same flask followed by 28 mL Carbon tetrachloride and 2 mL ortho- Phosphoric acid. This solution was shaken well to dissolve the reagent. This solution was transferred to 500 mL separating funnel and 200 mL water was added. Extraction was done by shaking well. The aqueous layer was separated. This solution was used for preparation of diluent.Diluent2, 4-DNPH solution: Acetonitrile (3:2).Preparation of Blank10 mL diluent and 6 mL water was taken into 20 mL volumetric flask. This flask was kept for mechanical stirring for 30 min. Volume made upto the mark with water and kept aside for 1 hr. standing.Standard Stock Solution205 mg of formaldehyde, 37% (Formalin) was weighed in 250 mL volumetric flask. Volume made upto 250 mL with water. 10 mL of this solution diluted to 100 mL with water. Transferred 1 mL of resultant solution to 100 mL volumetric flask and diluted up to the mark with water. Further, 1.0 mL of above solution is diluted to 100 mL with water.Preparation of Formaldehyde Standard SolutionIn 50 mL volumetric flask, 18 mL diluent and 2 mL of Standard stock solution of formaldehyde solution was taken. This flask was kept for mechanical stirring for 30 min. Volume made upto the mark with water and kept for 1 hr. standing (concentration of formaldehyde approx. 0.03 ppm).Preparation of Sample solution200 mg of crushed sample weighed and transferred in 50 mL volumetric flask. 18 mL of diluent and 2 mL of water was added to the flask. This flask was kept for mechanical stirring for 30 min. Volume made upto the mark with water and kept aside for 1 hr. standing.Note: Sample preparation can be adjusted to obtained the area of sample solution within range of calibration curve.Derivatization reaction used is 2,4-dinitrophenylhydrazine2,4-Dinitrophenylhydrazine used to detect the carbonyl functionality of formaldehyde. Presence of formaldehyde is indicated by a yellow or red precipitate (known as a dinitrophenyl hydrazone). Thus, 2, 4-DNP was used as a diluent for sample preparation. Note: Store Standard stock solutions, Standard solution and Sample solution at 8°C, immediately after preparation.Method Development and Method ValidationThe most widely used methods for the detection of formaldehyde are based on spectrophotometry, but other methods, such as colorimetry, fluorimetry, high-performance liquid chromatography, polarography, gas chromatography are also used. Organic and inorganic components in food products, other aldehydes and amines, can interfere with these methods of detection. ELSD is preferred for development of method for food products as it is considered as a universal detector due to the specificity of the detection method, which is based on the scattering of laser light on the non-volatile analyte particles. ELSD detector has advantage over other detectors for food samples analysis as other components, colors, additives, preservatives do not interfere in quantification, which is not possible with Gas chromatography detection and UV spectrophotometric detection [7]. Different HPLC columns with Octadecylsilane stationary phase were evaluated. However, C18, 250 x 4.6 mm, 5 µm column from Inertsil brand was found suitable. Similarly, different mobile phase compositions were tried but satisfactory separation and symmetrical peak was obtained by using gradient elution with selected composition of diluted formic acid by using gradient mode [8]. Formaldehyde do not have chromophore; quantification is done with derivatization technique. 2,4- dinitrophenyl hydrazine is used as derivatization reagent. Formaldehyde forms a hydrazone derivative upon reaction with 2,4-dinitrophenylhydrazine [9]. Use of derivatization technique enabled enhanced response for formaldehyde to ensure trace level detection. The reaction between 2, 4- Dinitrophenyl hydrazine and formaldehyde is shown in figure 1.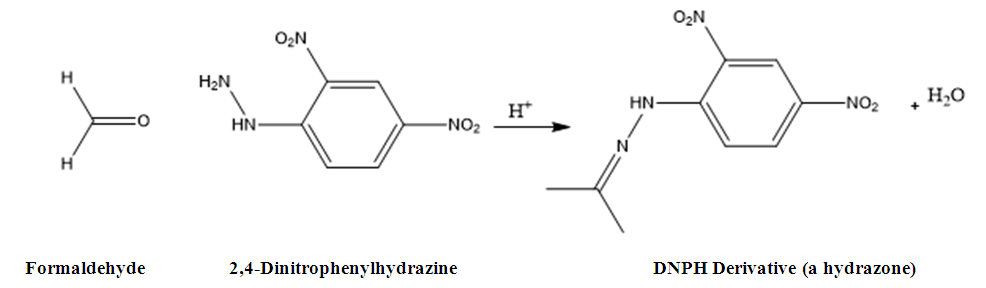 | Figure 1. Reaction between 2, 4-Dinitrophenylhydrazine and formaldehyde |
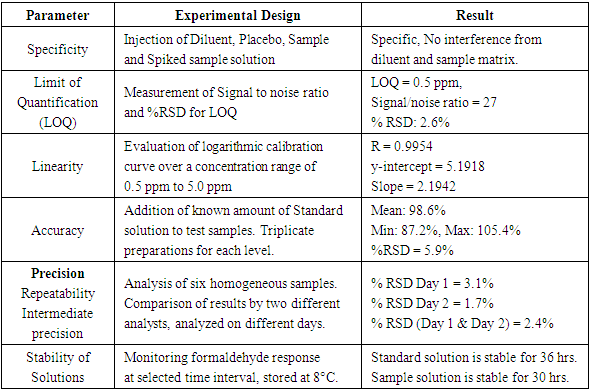
Developed method is subjected to method validation. The sensitivity of the method is challenged by injecting lower concentration of formaldehyde. Analytical validation is performed for selected validation parameters Specificity, Limit of Quantification (LOQ), Linearity, Accuracy, Precision and Solution stability in accordance with ICH Q2 (R1) guideline [10,11]. Method validation experimental design and results summary is reported in Table 2. Table 2. Method Validation Experimental Design and Results Summary
 |
| |
|
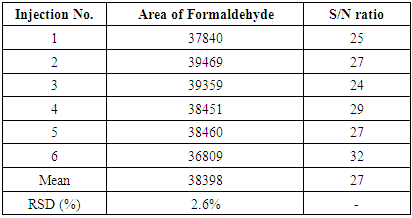
Response obtained with ELSD detector is nonlinear. The concentration of unknown samples is obtained by taking the logarithm of the instrument readings, computing the corresponding logarithms of the concentrations from the calibration equation, then taking the anti-log to obtain the concentration. The linearity of the method is tested over a concentration range of 0.5 ppm (LOQ) to 5.0 ppm. Limit of Quantification (LOQ) results are reported in Table 3.Table 3. Precision at Limit of Quantification (LOQ)
 |
| |
|
A typical HPLC chromatograms of Blank, Standard and Food sample are shown in Figure 2, Figure 3 and Figure 4 respectively. Chromatogram for Limit of Quantification (LOQ) is represented in Figure 5. Linearity results are reported in Table 4 and a logarithmic linearity plot is represented in Figure 6. Results for Accuracy, Precision and Solution stability are reported in Table 5, Table 6, Table 7 and Table 8 respectively. | Figure 2. Chromatogram of Blank Solution |
 | Figure 3. Chromatogram of formaldehyde Standard |
 | Figure 4. Chromatogram of food sample |
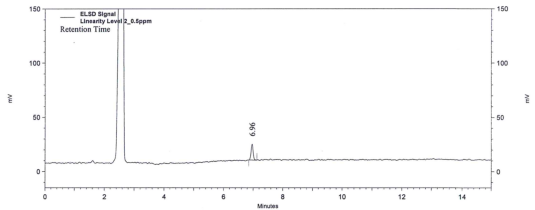 | Figure 5. Chromatogram of Limit of Quantification (LOQ) |
Table 4. Linearity regression analysis data
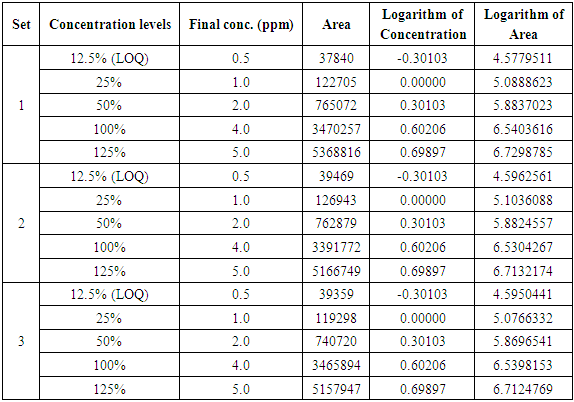 |
| |
|
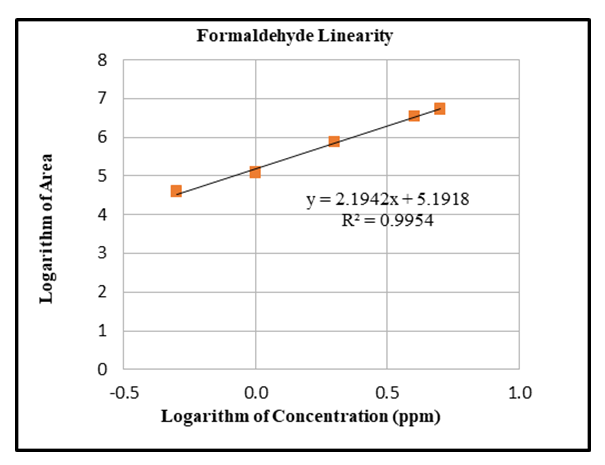 | Figure 6. Linearity_Logarithmic Calibration curve for formaldehyde |
Table 5. Accuracy regression analysis data
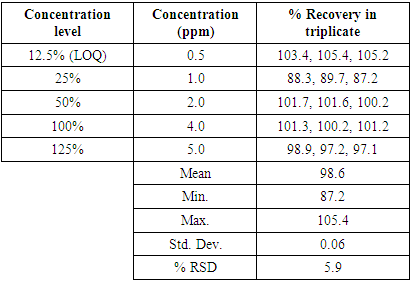 |
| |
|
Table 6. Statistical evaluation of the Formaldehyde Content data obtained in Method Precision (Day 1) and Intermediate precision (Day 2)
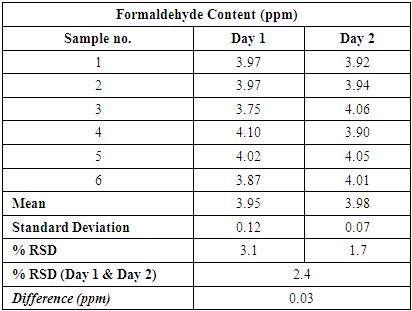 |
| |
|
Table 7. Results of Standard Solution stability at 8°C
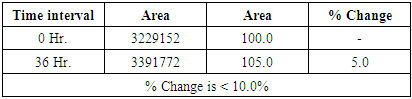 |
| |
|
Table 8. Results of Sample Solution stability at 8°C
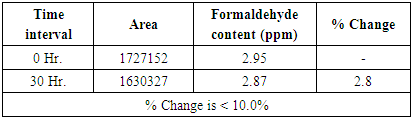 |
| |
|
3. Results and Discussion
The developed HPLC based ELSD method is used for quantification of trace level formaldehyde in selected baby food products, picked from market. Samples were selected to include most consumed brands in various food categories such as Biscuits, Jam, Ketchup, Dairy product, and Food supplements. The higher sensitivity of the method is indicated by sharpness of the peak (higher signal to noise ratio) and the concentration of the sample. As the evaporative light scattering detector response increases exponentially with an increase in formaldehyde concentration, both the sharpness of the peak and higher area response provides accuracy and reproducibility for quantification of formaldehyde at trace level. Selected baby food products contain additives such as food colours, flavours and preservatives. Presence of these additives did not affect the performance, accuracy and sensitivity of the method as ELSD detection is unique and eliminates possibility of interference from these components unlike UV detection or GC FID detection. The various randomly selected baby food products available in market are analysed for formaldehyde content. The results for selected baby food are reported in table 9.Table 9. Results of Baby food
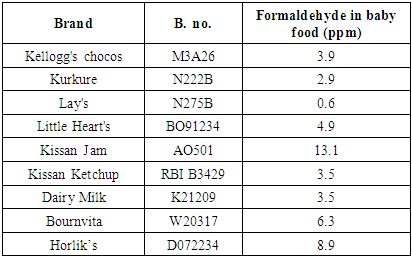 |
| |
|
Figure 7 represents comparative analysis results for formaldehyde content in selected baby food.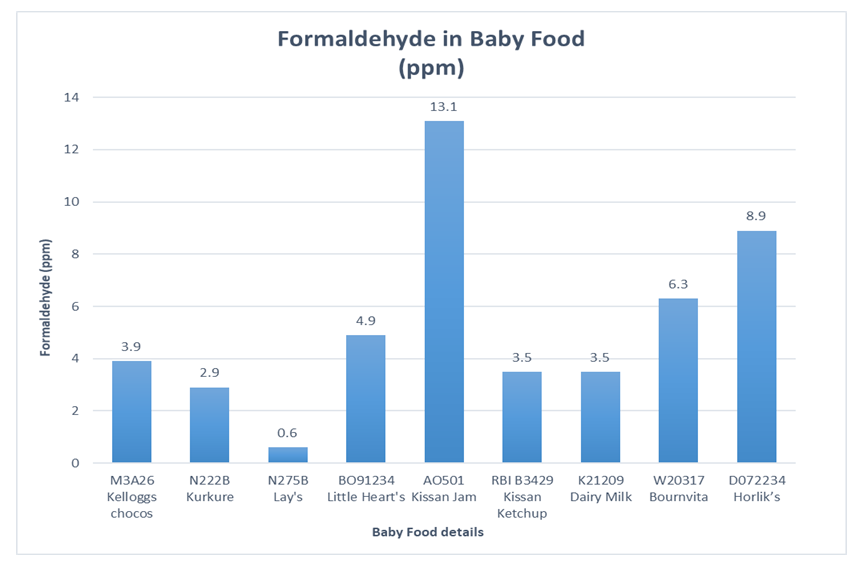 | Figure 7. Formaldehyde in Baby food |
4. Conclusions
This paper presents the development and validation of a simple High Performance Liquid Chromatography based ELSD method suitable for the analysis of formaldehyde in selected baby foods available in market. It is demonstrated that the analytical procedure developed is sensitive, accurate and precise with good stability in selected solvent, as results for selected validation parameters meet the requirements of ICH Q2 (R1) guideline. Specificity of the method was not compromised due to presence of additives such as food colours, flavours and preservatives in selected food products, which proved the advantages of ELSD detector over other commonly used analytical techniques such as Gas chromatography and UV spectrophotometry. Results observed for test samples were precise and reproducible. A very good linear fit of log ELSD response against log formaldehyde concentration is observed. The formaldehyde derivatization reaction with 2,4-dinitrophenylhydrazine and detection by ELSD detector are expected to be applicable to analysis of formaldehyde in other test samples such as various Food products, Cosmetic products, Consumer products and Pharmaceutical preparations available in market as long as these products disintegrates or are soluble in water. Sample preparation procedure can be modified including diluent used, to ensure complete disintegration of sample matrix. Further, this study has revealed presence of higher amount of formaldehyde content in some of the tested food products. In such case, Quantitative determination of the formaldehyde levels in food products is very important as chronic exposure to Formaldehyde can result is serious health hazards. Accurate results obtained by using developed method will enable control of formaldehyde content in various Food products, Cosmetic products, Consumer products and Pharmaceutical preparations within allowable tolerance levels defined by Occupational Health and Safety Administration (OSHA) and World Health Organization (WHO).
ACKNOWLEDGEMEMNTS
I wish to acknowledge the guidance provided by my research guide Dr. Sushama R. Ambadekar. I would also like to show my deep appreciation to technical and support staff of Institute of Science, Mumbai for helping me in conducting my work for this research paper.
References
| [1] | Occupational Safety and Health Administration (OSHA). Occupational Safety and Health Standards, Toxic and Hazardous Substances. Code of Federal Regulations 29 CFR1910.1048. 1998. |
| [2] | Concise International Chemical Assessment Document 40, Formaldehyde; World Health Organization, Geneva 2002. |
| [3] | Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC), Use of formaldehyde as a preservative in food additives Question Nº EFSA Q-2005-032. The EFSA Journal, 2006; 415: 1-10. |
| [4] | Food Safety Focus– Formaldehyde in Food. Centre for Food Safety, The Government of the Hong Kong Special Administrative Region, 6th Issue, January 2007. |
| [5] | U.S. Environmental Protection Agency. Health and Environmental Effects Profile for Formaldehyde. EPA/600/x-85/362. Environmental Criteria and Assessment Office, Office of Health and Environmental Assessment, Office of Research and Development, Cincinnati, OH. 1988. |
| [6] | U.S. Department of Health and Human Services. Registry of Toxic Effects of Chemical Substances (RTECS, online database). National Toxicology Information Program, National Library of Medicine, Bethesda, MD. 1993. |
| [7] | Vicente L. Cebolla*, Luis Membrado, Jesus Vela, and Ana C Ferrarido; Evaporative Light-Scattering Detection in the Quanti tative Analysis of Semivolatile Polycyclic Aromatic Compounds by High-Performance Liquid Chromatography. Journal of Chromatographic Science, Vol. 35, April 1997. |
| [8] | Lloyd R. Snyder, Joseph L. Glajch, and Joseph J. Kirkland; Practical HPLC Method Development, 2nd Ed, Published March 17th 1997, Wiley-Interscience. |
| [9] | ZHAO J. FAN B. 2006. Spectrophotometric Determination Method of Formaldehyde [J]. Guangdong Trace Elements Science, 13(2): 17-22. ISSN 1006-446X. |
| [10] | Validation of Analytical Procedures: Text and Methodology Q2 (R1). |
| [11] | Validating Chromatographic Methods: A Practical Guide, David M. Bliesn. |


 Detector ParametersDetection: Agilent 1260 Infinity Evaporative Light Scattering DetectorEvaporator temperature: 60°C Nebulizer temperature: 50°C Gas Flow rate: 1.60 SLMData rate: 80 HzLED Intensity: 100%PMT gain: 10.0Preparation of 2, 4-DNPH Solution833 mg of 2, 4-DNPH was weighed and transferred in 200 mL volumetric flask. 170 mL of Acetonitrile added to the same flask followed by 28 mL Carbon tetrachloride and 2 mL ortho- Phosphoric acid. This solution was shaken well to dissolve the reagent. This solution was transferred to 500 mL separating funnel and 200 mL water was added. Extraction was done by shaking well. The aqueous layer was separated. This solution was used for preparation of diluent.Diluent2, 4-DNPH solution: Acetonitrile (3:2).Preparation of Blank10 mL diluent and 6 mL water was taken into 20 mL volumetric flask. This flask was kept for mechanical stirring for 30 min. Volume made upto the mark with water and kept aside for 1 hr. standing.Standard Stock Solution205 mg of formaldehyde, 37% (Formalin) was weighed in 250 mL volumetric flask. Volume made upto 250 mL with water. 10 mL of this solution diluted to 100 mL with water. Transferred 1 mL of resultant solution to 100 mL volumetric flask and diluted up to the mark with water. Further, 1.0 mL of above solution is diluted to 100 mL with water.Preparation of Formaldehyde Standard SolutionIn 50 mL volumetric flask, 18 mL diluent and 2 mL of Standard stock solution of formaldehyde solution was taken. This flask was kept for mechanical stirring for 30 min. Volume made upto the mark with water and kept for 1 hr. standing (concentration of formaldehyde approx. 0.03 ppm).Preparation of Sample solution200 mg of crushed sample weighed and transferred in 50 mL volumetric flask. 18 mL of diluent and 2 mL of water was added to the flask. This flask was kept for mechanical stirring for 30 min. Volume made upto the mark with water and kept aside for 1 hr. standing.Note: Sample preparation can be adjusted to obtained the area of sample solution within range of calibration curve.Derivatization reaction used is 2,4-dinitrophenylhydrazine2,4-Dinitrophenylhydrazine used to detect the carbonyl functionality of formaldehyde. Presence of formaldehyde is indicated by a yellow or red precipitate (known as a dinitrophenyl hydrazone). Thus, 2, 4-DNP was used as a diluent for sample preparation. Note: Store Standard stock solutions, Standard solution and Sample solution at 8°C, immediately after preparation.Method Development and Method ValidationThe most widely used methods for the detection of formaldehyde are based on spectrophotometry, but other methods, such as colorimetry, fluorimetry, high-performance liquid chromatography, polarography, gas chromatography are also used. Organic and inorganic components in food products, other aldehydes and amines, can interfere with these methods of detection. ELSD is preferred for development of method for food products as it is considered as a universal detector due to the specificity of the detection method, which is based on the scattering of laser light on the non-volatile analyte particles. ELSD detector has advantage over other detectors for food samples analysis as other components, colors, additives, preservatives do not interfere in quantification, which is not possible with Gas chromatography detection and UV spectrophotometric detection [7]. Different HPLC columns with Octadecylsilane stationary phase were evaluated. However, C18, 250 x 4.6 mm, 5 µm column from Inertsil brand was found suitable. Similarly, different mobile phase compositions were tried but satisfactory separation and symmetrical peak was obtained by using gradient elution with selected composition of diluted formic acid by using gradient mode [8]. Formaldehyde do not have chromophore; quantification is done with derivatization technique. 2,4- dinitrophenyl hydrazine is used as derivatization reagent. Formaldehyde forms a hydrazone derivative upon reaction with 2,4-dinitrophenylhydrazine [9]. Use of derivatization technique enabled enhanced response for formaldehyde to ensure trace level detection. The reaction between 2, 4- Dinitrophenyl hydrazine and formaldehyde is shown in figure 1.
Detector ParametersDetection: Agilent 1260 Infinity Evaporative Light Scattering DetectorEvaporator temperature: 60°C Nebulizer temperature: 50°C Gas Flow rate: 1.60 SLMData rate: 80 HzLED Intensity: 100%PMT gain: 10.0Preparation of 2, 4-DNPH Solution833 mg of 2, 4-DNPH was weighed and transferred in 200 mL volumetric flask. 170 mL of Acetonitrile added to the same flask followed by 28 mL Carbon tetrachloride and 2 mL ortho- Phosphoric acid. This solution was shaken well to dissolve the reagent. This solution was transferred to 500 mL separating funnel and 200 mL water was added. Extraction was done by shaking well. The aqueous layer was separated. This solution was used for preparation of diluent.Diluent2, 4-DNPH solution: Acetonitrile (3:2).Preparation of Blank10 mL diluent and 6 mL water was taken into 20 mL volumetric flask. This flask was kept for mechanical stirring for 30 min. Volume made upto the mark with water and kept aside for 1 hr. standing.Standard Stock Solution205 mg of formaldehyde, 37% (Formalin) was weighed in 250 mL volumetric flask. Volume made upto 250 mL with water. 10 mL of this solution diluted to 100 mL with water. Transferred 1 mL of resultant solution to 100 mL volumetric flask and diluted up to the mark with water. Further, 1.0 mL of above solution is diluted to 100 mL with water.Preparation of Formaldehyde Standard SolutionIn 50 mL volumetric flask, 18 mL diluent and 2 mL of Standard stock solution of formaldehyde solution was taken. This flask was kept for mechanical stirring for 30 min. Volume made upto the mark with water and kept for 1 hr. standing (concentration of formaldehyde approx. 0.03 ppm).Preparation of Sample solution200 mg of crushed sample weighed and transferred in 50 mL volumetric flask. 18 mL of diluent and 2 mL of water was added to the flask. This flask was kept for mechanical stirring for 30 min. Volume made upto the mark with water and kept aside for 1 hr. standing.Note: Sample preparation can be adjusted to obtained the area of sample solution within range of calibration curve.Derivatization reaction used is 2,4-dinitrophenylhydrazine2,4-Dinitrophenylhydrazine used to detect the carbonyl functionality of formaldehyde. Presence of formaldehyde is indicated by a yellow or red precipitate (known as a dinitrophenyl hydrazone). Thus, 2, 4-DNP was used as a diluent for sample preparation. Note: Store Standard stock solutions, Standard solution and Sample solution at 8°C, immediately after preparation.Method Development and Method ValidationThe most widely used methods for the detection of formaldehyde are based on spectrophotometry, but other methods, such as colorimetry, fluorimetry, high-performance liquid chromatography, polarography, gas chromatography are also used. Organic and inorganic components in food products, other aldehydes and amines, can interfere with these methods of detection. ELSD is preferred for development of method for food products as it is considered as a universal detector due to the specificity of the detection method, which is based on the scattering of laser light on the non-volatile analyte particles. ELSD detector has advantage over other detectors for food samples analysis as other components, colors, additives, preservatives do not interfere in quantification, which is not possible with Gas chromatography detection and UV spectrophotometric detection [7]. Different HPLC columns with Octadecylsilane stationary phase were evaluated. However, C18, 250 x 4.6 mm, 5 µm column from Inertsil brand was found suitable. Similarly, different mobile phase compositions were tried but satisfactory separation and symmetrical peak was obtained by using gradient elution with selected composition of diluted formic acid by using gradient mode [8]. Formaldehyde do not have chromophore; quantification is done with derivatization technique. 2,4- dinitrophenyl hydrazine is used as derivatization reagent. Formaldehyde forms a hydrazone derivative upon reaction with 2,4-dinitrophenylhydrazine [9]. Use of derivatization technique enabled enhanced response for formaldehyde to ensure trace level detection. The reaction between 2, 4- Dinitrophenyl hydrazine and formaldehyde is shown in figure 1.






 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML







