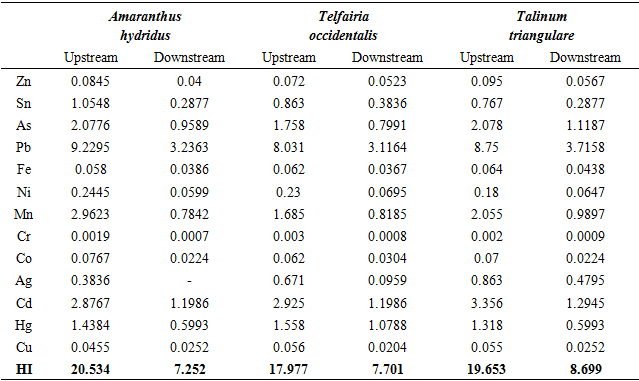
| [1] | Afolayan, O.S., Ogundele, F.O. and Odewumi, S.G. (2012). Hydrological implication of solid waste disposal on groundwater quality in urbanized area of Lagos State, Nigeria. International Journal of Applied Science and Technology, 2(5): 74-82. |
| [2] | Kola-Olusanya, A. (2003). Impact of municipal solid wastes on underground water sources in Nigeria. European Scientific Journal, 8(11): 1-19. |
| [3] | Okalebo, S.E., Opata, G.P. and Mwasi, B.N. (2014). An analysis of the household solid waste generation patterns and prevailing management practices in Eldoret town, Kenya. International Journal of Agricultural Policy and Research, 2(2): 76. |
| [4] | Zhang, D.Q., Tan, S.K. and Gersberg, R.M. (2010). Municipal solid waste management in China: status, problems and challenges. J. Environ. Manage., 91(8): 1623-1633. |
| [5] | Ogwueleka, T. C. (2009). Municipal Solid Waste Characteristics and Management in Nigeria. Environmental Health, Science and Engineering, 6(3): 173-180. |
| [6] | Amadi, A. N., Olasehinde, P. I., Okosun, E. A., Okoye, N. O., Okunlola, I. A., Alkali, Y. B. and Dan-Hassan, M. A. (2012). A comparative study on the impact of Avu and Ihie dumpsites on soil quality in Southeastern Nigeria. American Journal of Chemistry, 2(1): 17-23. |
| [7] | Al-Sabahi E., Abdul Rahim S., Wan Zuhairi W.Y., Al Nozaily F. and Alshaebi, F. (2009). The characteristics of leachate and groundwater pollution at municipal solid waste landfill of Ibb City, Yemen. American Journal of Environmental Sciences, 5: 256–266. |
| [8] | Ali, S.M., Pervaiz, A., Afzal, B., Hamid,N. and Yasmin, A.(2014). Open dumping of municipal solid waste and its hazardous impacts on soil and vegetation diversity at waste dumping sites of Islamabad city. Journal of King Saud University–Science, 26: 59–65. |
| [9] | Banar, M., Aysun, O. and Mine, K. (2006). Characterization of the leachate in an urban landfill by physicochemical analysis and solid phase micro extraction. GC/MS. Environ. Monitor. Assess., 121: 439 – 459. |
| [10] | Tahri, F., Benya, M., Bounakla, E.I. and Bilal, J.J. (2005). Multivariate analysis of heavy metal in soils, sediments and water in the region of Meknes, Central Morocco. Environ. Monitor. Asses., 102: 405–417. |
| [11] | Amadi, A.N., Ameh, M.I. and Jisa, J. (2010). The impact of dump-sites on groundwater quality in Markurdi Metropolis, Benue State. Journal of Natural and Applied Sciences, 11(1): 90–102. |
| [12] | Ogbonna, D. N., Simeon, N., Chilaka, S. N. and Gobo, A. E. (2008). Effects of poor waste disposal practices and perennial flooding on groundwater in Port Harcourt. Journal of Environmental Science, 4(2): 59-65. |
| [13] | Shaylor, H., McBride, M. and Harrison, E. (2009). Sources and Impacts of contaminants in Soil. Cornell Waste Management Institute. p 35. |
| [14] | Akinro, A. O., Ikumawoyi, O. B., Olotu, Y. and Ologunagba, M.M. (2012). Environmental impacts of polyethylene generation and disposal in Akure City, Nigeria. Global Journal of Science Frontier Research Agriculture and Biology, 12(3): 1-8. |
| [15] | Gurunadha Rao, V. V. S., Dhar, R. L. and Subrahmanyam, K. (2011). Assessment of contaminant migration in groundwater from industrial development area, Medak Andhra Pradesh, India. National Geophysical Research Institute (Council of Scientific Industrial Research), 128(7): 369 – 389. |
| [16] | Erah, P. O., Akujieze, C. N. and Oteze, G. E. (2002). The quality of groundwater in Benin City: A baseline on inorganic chemicals and microbial contamination of health importance in boreholes and open wells. Trop. J. Pharm. Res., pp: 75 – 82. |
| [17] | Uwah, E.I., Ndahi, N.P. and Ogugbuaja, V.O. (2009). Study of the levels of some agricultural pollutants in soils, and water leaf (Talinum triangulare) obtained in Maiduguri, Nigeria. Journal of applied Science and Environmental Sanitation, 4(2): 71-78. |
| [18] | Gomiero, T. (2018). Food quality assessment in organic vs. conventional agricultural produce: findings and issues. Appl. Soil Ecol., 123:714-728. |
| [19] | Arora, M., Kiran, B., Rani, S., Rani, A., Kaur, B. and Mittal, N. (2008). Heavy metal accumulation in vegetables irrigated with water from different sources. Food Chemistry, 111(4): 811-815. |
| [20] | Shaheen, N., Irfan, N.M., Khan, I.N., Islam, S., Islam, M.S. and Ahmed, M.K. (2016). Presence of heavy metals in fruits and vegetables: health risk implications in Bangladesh. Chemosphere, 152: 431-438. |
| [21] | Jarup, L. (2003). Hazards of heavy metal contamination. British Medical Bulletin, 68(1): 167-182. |
| [22] | Stephen, O. M., Chinedum, O. M., Segun, A. A and David, O. M. (2013). The physicochemical effect of leachates on ground water within Okpuno-Egbu Umudim Dumpsite Nnewi, Anambra State Nigeria. Daffodil International University Journal of Science and Technology, 8(2): 25. |
| [23] | Tsafe, A. I., Hassan, L. G., Sahabi, D. M., Alhassan, Y. and Bala, B.M. (2012). Evaluation of Heavy Metals Uptake and Risk Assessment of Vegetables Grown in Yargadama of Northern Nigeria, Journal of Basic and Applied Scientific Research, 2(7): 6708-6714. |
| [24] | Agrawal, M., Singh, A., Sharma, R.K. and Marshall, F.M. (2010). Risk assessment of heavy metal toxicity through contaminated vegetables from waste water irrigated area of Varanasi, India. International Society for Tropical Ecology, 51(2S): 375-387. |
| [25] | FAO/WHO, (2004). Fruit and vegetables for health. Kobe, Japan: Report of Joint FAO/WHO Workshop. p 1–45. |
| [26] | USEPA (2001). Risk Assessment Guidance for Superfund: Volume III - Part A, Process for conducting probabilistic risk assessment. EPA 540 – R – 02 – 002. www.epa.gov/superfund/RAGS3A/index.htm. |
| [27] | Ramteke, S., Sahu, B.L., Dahariya, N.S., Patel, K.S., Blazhev, B. and Matini, L. (2016) Heavy metal contamination of leafy vegetables. Journal of Environmental Protection, 7: 996-1004. http://dx.doi.org/10.4236/ jep.2016.77088. |
| [28] | USEPA (2007). Integrated Risk information system databased. Philadelphia PA; Washington, DC, USA. |
| [29] | USEPA (2015). Human health risk assessment: risk-based concentration table. http://www.epa.gov/reg3hwmd/risk/human/rb-concentration table/GenericTables/index.htm. |
| [30] | Guerra, F., Trevizam, A.R., Muraoka, T., Marcante, N.C. and Canniatti-Brazaca, S.G. (2012). Heavy metals in vegetables and potential risk for human health. Journal of Scientia Agricola, 69(1): 54-60. |
| [31] | Khan, S. A., Khan, L., Hussain, I., Marwat, K. B., and Ashtray, N. (2008). Profile of heavy metals in selected medicinal plants. Pakistan Journal of Weed Science Research, 14(1–2): 101–110. |
| [32] | Fosu-Mensah. B.Y., Addae, E., Yirenya-Tawiah, D. and Nyame, F. (2017). Heavy metals concentration and distribution in soils and vegetation at Korle Lagoon area in Accra, Ghana. Cogent Environmental Science, 3 (1): 1-14. |
| [33] | FAO/WHO, (2017). Joint FAO/WHO food standards programme codex committee on contaminants in foods. Eleventh Session. Codex Alimentarius Commission, pp 1-162. |
| [34] | Jabeen, S., Shah, M. T., Khan, S. and Hayat, M. Q. (2010). Determination of major and trace elements in ten important folk therapeutic plants of Haripur basin, Pakistan. Journal of Medicinal Plants Research, 4(7): 559–566. |
| [35] | Maobe, M. A. G., Gatebe, E., Gitu, L. and Rotich, H. (2012). Profile of heavy metals in selected medicinal plants used for the treatment of diabetes, malaria and pneumonia in Kisii region, Southwest Kenya. Global Journal of Pharmacology, 6(3): 245–251. |
| [36] | Knezevic, M., Stankovic, D., Krstic, B., Nikolic, M. S., and Vilotic, D. (2009). Concentrations of heavy metals in soil and leaves of plant species Paulownia elongata and Paulownia fortunei. African Journal of Biotechnology, 8(20): 5422–5429. |
| [37] | Fagbote, E. O. and Olanipekun, E. O. (2010). Evaluation of the status of heavy metal pollution of soil and plant (Chromolaena odorata) of Agbabu bitumen deposit area, Nigeria. American-Eurasian Journal of Scientific Research, 5(4): 241–248. |
| [38] | Cui, Y.J., Zhu, Y.G., Zhai, R.H., Chen, D.Y., Huang, Y.Z., Qui, Y. and Liang, J.Z. (2004). Transfer of metals from near a smelter in Nanning, China. Environmental International, 30(6): 785-791. |
| [39] | Abah, J., Abdurahaman, F.I., Ndahi, N.P. and Ogugbuaja, V O. (2012). Effects of inorganic fertilizers and herbicides on levels of some heavy metals in soils and grains of rice grown in selected areas of Benue State, Nigeria. International Journal of Applied and Resources Technology, 1: 160-166. |
| [40] | Kachenko, A.G. and Singh, B. (2006). Heavy metals contamination in vegetables grown in urban and metal smelter contaminated sites in Australia. Water Air Soil Pollution, 169: 101-123. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML