Jean Bruno Mokoko 1, Victor N'goka 2, Enoua Guy Crépin 2, Lyi Phassole Loemba Mbatchi 2
1Faculty of Health Sciences, Marien N'Gouabi University, Brazzaville, Republic of Congo
2Faculty of Science and Technology, Marien N'Gouabi University, Brazzaville, Republic of Congo
Correspondence to: Jean Bruno Mokoko , Faculty of Health Sciences, Marien N'Gouabi University, Brazzaville, Republic of Congo.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
This study focuses on the analysis of coolants in automotive engines for sale in Brazzaville. The parameters; pH, refractive index, densities and spectrum in the ultraviolet were measured. It follows that the colours of the coolants for sale in Brazzaville are random, the refractive indices are comparable to that of water except for one product, 50% of coolants have acidic pH and are known to degrade engines, 85% appear to be stained with paint dyes for sale in hardware stores, or those of the sky and/ or ice cream on sale in the markets. These liquids do not contain ethylene glycol except for one product. And, generally speaking, coolants for sale in Brazzaville are not compliant.
Keywords:
Analysis, Coolant, Brazzaville
Cite this paper: Jean Bruno Mokoko , Victor N'goka , Enoua Guy Crépin , Lyi Phassole Loemba Mbatchi , Physico-chemical Analysis of Engine Coolants for Sale in Brazzaville, American Journal of Chemistry, Vol. 10 No. 1, 2020, pp. 6-10. doi: 10.5923/j.chemistry.20201001.02.
1. Introduction
A coolant is used in a generally closed circuit to remove heat [1]. These liquids are mainly composed of water, ethylene or propylene glycol [2,3,4] and anticorrosive additives based on organic or inorganic inhibitors [5,6,7,8]. In recent years, the Congo is open to all kinds of imports. There is no structured system for controlling import products in the Congo. It is therefore becoming imperative to control these import products in order to protect consumers in any way. CHIRED-CONGO (Chemistry Research and Pharmaceutical Development) in its part to serve the society has already carried out a study on Dakin’s solutes for sale in Brazzaville with surprising results [9,10]. Thus our study concerns the verification of the conformity or not of coolant liquids for sale in Brazzaville by means of physico-chemical analyses.
2. Materials and Method
2.1. Material
Equipment: survey sheets; Areometer; pH meter model HANNA instrument, HI 8014, Spectrophotometer UV-2700 spectrophotometer with controlled; refractometer ABBE model NOVEX HOLLAND; rotary evaporator; Analytical chromatography plates and accessories. Chemicals: coolants, 5 blue, green, red, pink and yellow food colours, 4 blue, yellow, red and green paint colours, acetone, distilled water, butan-2-ol solution, glacial acetic acid, potassium permanganate, buffer solutions at pH=4.01; 7.21 and 9.21.
2.2. Methods
Measurement of the colour The colors were appreciated by an eye exam.Measurement of refractive indexFor each coolant, the measurement was performed using an ABBE refractometer model NOVEX HOLLAND. For each value of the measured refractive index we made a correction using the formula n= n_D- 0,00045(T-20) with n_D the measured refractive index and T the ordinary temperature.pH measurementFor each coolant, the measurement was performed with a pH meter model HANNA. We carried out a rapid test by calibrating the pH meter with the pH buffer solution equal to 7.21 and then measured the pH of each liquid. Based on the range of pH values obtained between 3.71 and 9.28 and the buffer solutions present, we grouped the liquids into three groups:• in the first group with a pH between 3,71 and 3,96. The pH was measured by calibrating the pH meter with the pH buffer solution equal to 4,01;• the second group the liquids had a pH between 5.98 and 8.71. pH was measured by calibrating the pH meter with pH buffer solution equal to 7.21;• and the third group pH ranged from 9.13 to 9.28. For each liquid belonging to this group we measured the pH by calibrating the pH meter with the pH buffer solution equal to 9.21.Measurement of densityIt was carried out using a graduated hydrometer from 1000 to 1100 by plunging the hydrometer into the test piece containing the coolant.Measurement of evaporative residueThe evaporation of the solvent contained in each coolant was carried out using a rotary evaporator, distilling 50 ml of each coolant. The resulting residue was weighed and collected in the vials.Thin Layer Chromatography (TLC)The thin layer analyses were performed from the samples of each coolant to determine whether ethylene glycol was present. This CCM was made on silica gel with the aluminum plate. The eluent consisting of 90% butan-2-ol, 9% distilled water and 1% glacial acetic acid was used in the glass vessels previously saturated with eluent. The revelation was made with the potassium permanganate solution and the presence of ethylene glycol was highlighted by the presence of yellow stains and then Rf were calculated.Measurement of the absorption spectrum in UV-Visible The spectrum of each coolant was measured using the scanning spectrophotometer UV-2700 spectrophotometer and controlled by microprocessor. Since these liquids were previously classified in groups of identical colours, we were led to compare them with those of paint and candy dyes sold in Brazzaville.Preparation of solutionsFor each coolant, we diluted 5 ml of the liquid taken with a 5 ml pipete into a 100 ml volumetric flask. For each paint dye we mix a few drops of the dye in 50 ml of distilled water and then shake. For food dyes, we put a small amount of dye powder in 50 ml of distilled water and then shake.The spectrum of each prepared solution was measured by performing a wavelength scan between 190 and 1100 nm.
3. Results and Discussion
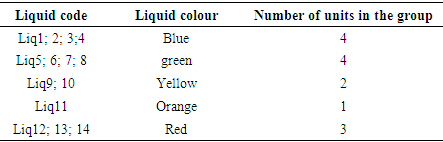
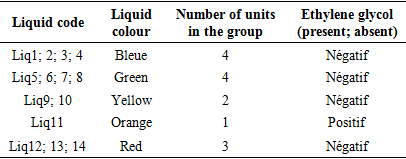
List and classification of colour coolantsTable 1. List and Classification of Coolants
 |
| |
|
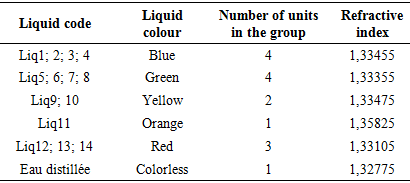
Table 1 presents the list and colour classification of coolants for sale in Brazzaville. This table shows that of the 14 liquids, 4 are blue, 4 are green, 2 are yellow, 1 is orange and 3 are red. In the bibliographical section PRESTONE has shown that the classification of coolants according to their colour is an outdated concept at present. From the point of view of the blue, green, yellow, orange and red colours, coolants for sale in Brazzaville do not provide any qualitative or quantitative information.Measurement of refractive index of coolantsTable 2. Refractive index of each coolant
 |
| |
|
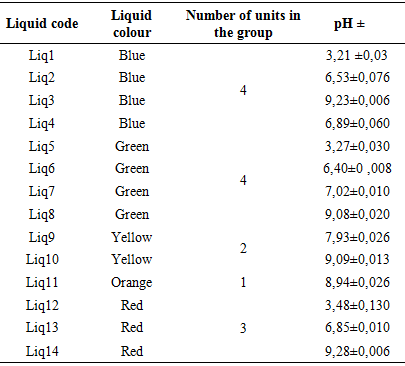
Table 2 shows the refractive index of each coolant. These results show that apart from Liq11, which has an index equal to 1.35825, all other liquids have an index close to the refractive index of water. According to the French standard NFR15601, the refractive indices of coolants range from 1.355 to 1.388. Again, only Liq11 can be considered as a coolant based on the refractive index.pH measurement of each coolantTable 3. pH of each coolant
 |
| |
|
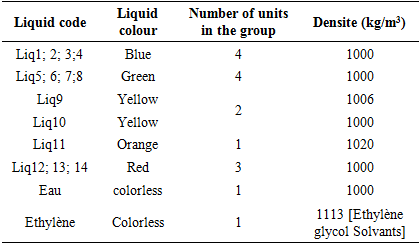
Table 3 shows the pH of each coolant. These results show that Liq1, Liq5 and Liq12 have a very acidic pH between 3.21 and 3.48. Liq6, Liq2, Liq13, Liq4 and Liq7 have respectively pH 6.40; 6.53; 6.85; 6.89 and 7.02; they can be considered liquid at neutral pH. The basic pH liquids are Liq9, Liq11, Liq8, Liq10, Liq3 and Liq14 because their pH ranges from 7.93 to 9.28. In accordance with FMVSS116, the pH of coolants should be between 7 and 11.5. For this standard only Liq7, Liq8, Liq9, Liq10, Liq11 and Liq14 have an acceptable pH. The Liq1, Liq2, Liq4, Liq5, Liq6, Liq12 and Liq13, which have a pH of less than 7, will be responsible for the occurrence of corrosion on the ferrous components of the engine as indicated in the bibliography of several reference models [11,12,13]. We see here, on a single parameter which is the pH that 7 of the 14 liquids are bad for users.Measuring the density of each coolantTable 4. Density of each coolant
 |
| |
|
Table 4 shows the density of each coolant in kg/m3. These results show that, apart from Liq9 and Liq11, all liquids have a density equal to 1,000 kg/m3. The literature [Ethylene glycol Solvents] has shown that ethylene glycol has a density of 1,113 kg/m3. The density of coolants should not be between water and ethylene glycol. From this table we can conclude that only Liq 9 and Liq11 would be likely to contain ethylene glycol. Qualitative analysis of coolants: ethylene glycol testingTable 5 and the photo below show the identification by thin-layer chromatography of ethylene glycol for each coolant. These results show that of the 14 coolants, only one would contain ethylene glycol. Liq11 can thus be considered as a coolant by simply identifying the essential constituent: ethylene glycol.Table 5. Ethylene glycol identification by CCM
 |
| |
|
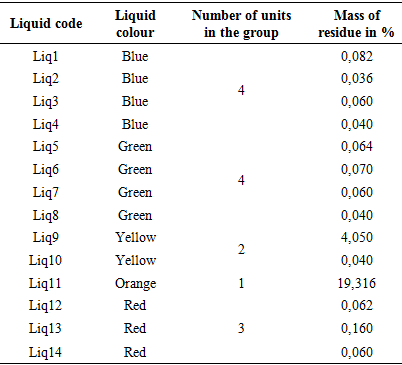
Measurement of the quantity ethylene glycolTable 6 shows the mass of residue obtained after evaporation to the rotary evaporator. All other liquids are found to have a residue percentage of less than 0.160. Only Liq9 and Liq11 have residues of 4.050 and 19.316, respectively. If it can be assumed that a coolant consists mainly of water and ethylene glycol, since the qualitative analysis presented in Table 5 has demonstrated the absence of ethylene glycol in Liq9. It can then be assumed that the mass of the residue of liquids that does not contain ethylene glycol results from additives as described in the bibliography (dyes, inhibitors, etc.). Under these conditions, it can be concluded that all other liquids do not contain ethylene glycol. The French standard NFR15601 specifies that the amount of ethylene glycol as a percentage in a coolant is between 20% and 50%. The result obtained for Liq11 presented in Table 6 further shows that only the liq11 coolant can be considered to conform to the standard in relation to its ethylene glycol quantity.Table 6. Measurement of the quantity ethylene glycol
 |
| |
|
Qualitative analysis of coolants: Absorption spectrum in ultraviolet-visibleThis group consists of three coolants which correspond to the spectra Liq1, Liq2 and Liq3. We have associated the spectrum of CA Blue reference sky dye for sale in the markets. The spectral characteristics are λmax (water) 312 nm, 408 nm and 628 nm for all four. We observe that these spectra are identical. It is probably this dye that is one of the constituents of these liquids. This coolant corresponds to the Liq4 spectrum. We have associated the spectrum of CP Blue paint dye for sale in hardware stores. The spectral characteristics are λmax (water) 344 nm, 636 nm and 716 nm for both. We observe that these spectra are identical. It is probably this dye that is one of the constituents of this liquid.This group is represented by three coolants which correspond to the spectra Liq5, Liq6 and Liq7. We have associated the spectrum of dye for sky reference CA Green, for sale in the markets: the spectral characteristics are λmax (water) 424 nm and 628 nm for the three and λmax (water) 316 nm, 418 nm and 628 nm for the fourth. When looking at the pH values, they are: 3.27; 6.40 and 7.02, we can say that these spectra are identical and that it is the simple fact of the changes in pH that would justify these slight differences. This also concludes with the use of a food colouring agent. This group of two spectra is Liq8 and CP Vert. We have associated the spectrum of dye for reference painter CP Verte for sale in hardware stores: The spectral characteristics are λmax (water) 316 nm and 648 nm. It is found that these spectra are identical and that it is probably this paint dye that is used for the manufacture of Liq8. This group consists only of a coolant corresponding to the Liq11 spectrum. This spectrum has no similarity to dye spectra. This coolant really has an originality on all the parameters objects of our study: Refractive index, high pH, density and amount of ethylene glycol. This group is represented by two coolants that correspond to the Liq12 and Liq13 spectra. The spectrum of CA Red reference sky dye for sale in hardware stores has the same spectral characteristics are λmax (water) 322 nm and 514 nm. Again, it is probably this food colouring that is used in the manufacture of Liq12 and Liq13. This group is represented by a coolant which corresponds to the Liq14 spectrum. The spectrum of dye for reference painter CP Rouge for sale in hardware stores, has the same spectral characteristics are λmax (water) 296 nm, 524 nm and 562 nm. Again, it is probably this paint dye that is one of the Liq14 constituents.
4. Conclusions
This study shows the colors of coolants for sale in Brazzaville are random. Their densities and refractive indices are comparable to that of water and do not conform to any standard except that of two products and do not contain ethylene glycol except one product. These results on coolants confirm the need to control the quality of all imported products.
Annexes
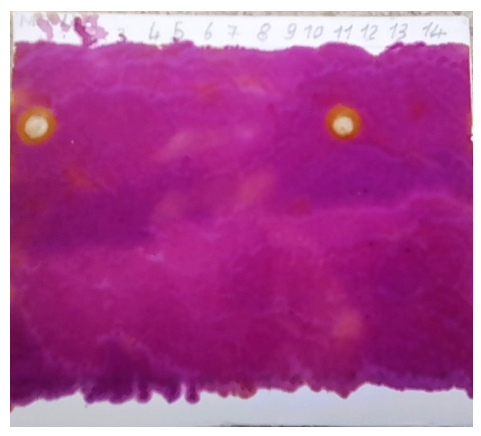 | Image of ethylene glycol chromatogram and Liq1-14. Silica gel plate on aluminium; Eluant: 90% butan-2-ol, 9% distilled water and 1% glacial acetic acid: Developer: Potassium permangante solution; Ethylene glycol Rf = 0.7. |
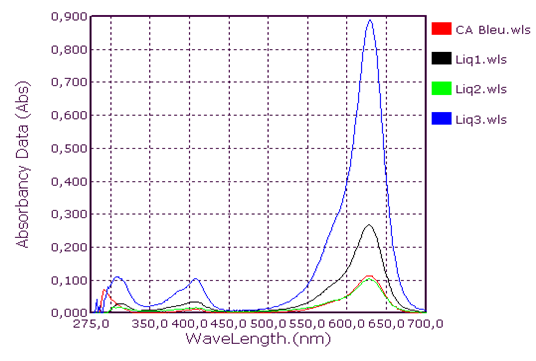 | Figure 1. Absorption spectrum of blue coolant |
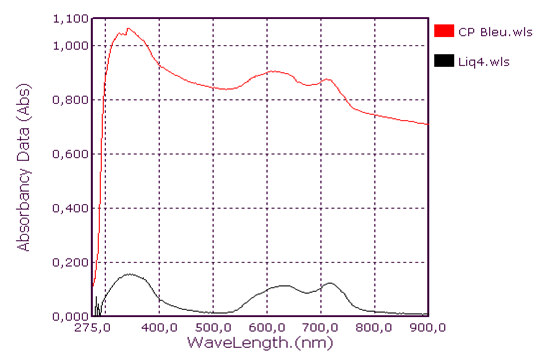 | Figure 2. Blue Coolant Absorption Spectrum: Liq4 |
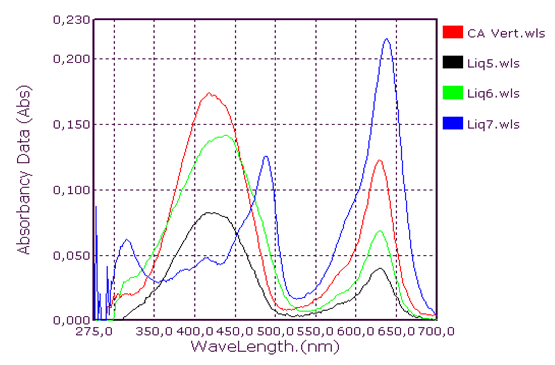 | Figure 3. Green Coolant Absorption Spectrum |
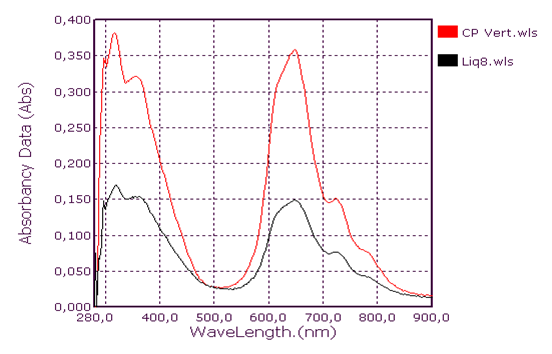 | Figure 4. Green Coolant absorption spectrum Liq8 |
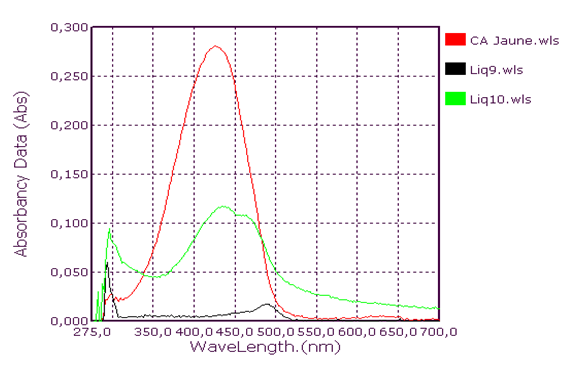 | Figure 5. Absorption spectrum of coolants Yellow |
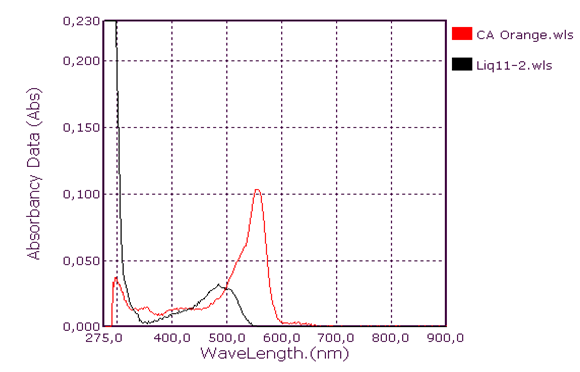 | Figure 6. Orange Coolant Absorption Spectrum |
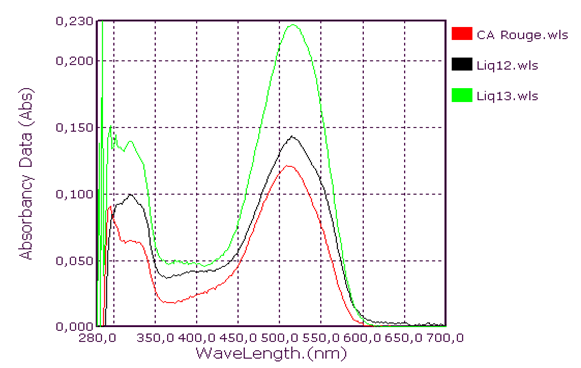 | Figure 7. Orange Coolant Absorption Spectrum |
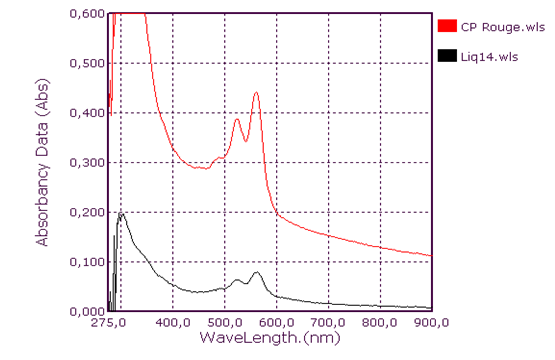 | Figure 8. Red coolant absorption spectrum: Liq14 |
References
| [1] | TRIBOLOGIK Infolettre, (mars 2014). "Analyses de base des liquides de refroidissement". |
| [2] | C.A Wurtz (1859), "Mémoire sur les glycols ou les alcools diatomiques", Annal. Chim. Phys, vol. 55, p.400-478. |
| [3] | C.A Wurtz (1859), "Synthèse du glycol avec l’oxyde d’éthylène et l’eau", vol. XLIX, p813-815. |
| [4] | The Royal Society of Chimistry Safety Chimical Data Sheet (1989), "éthylène glycol Solvent", p147-150. |
| [5] | E. H. Norman, “NACE Glossary of Corrosion Terms,” Materials Protection, Vol. 4, No. 1, 1965, p. 79. |
| [6] | CONSTANTIN Florina (2011), "étude de l’efficacité d’inhibiteurs de corrosion utilisés dans les liquides de refroidissement", Thèse de doctorat en cotutelle Franco-Roumaine, Université de Pitesti-Roumanie, p27. |
| [7] | Christian Fouché (2019), "Quel type de liquide de refroidissement pour mon Anglaise?" Informations techniques - Amicale véhicules anglais. |
| [8] | HAROUAKA Saad (2013), "influence de la température et de l’agitation du liquide de refroidissement sur la teneur à la corrosion des alliages d’aluminium utilisés dans l’industrie automobile", Mémoire du Magister, soutenu 27 décembre 2013; Université du 20 Août 1955-Skikda, République Algérienne Démocratique Et Populaire. |
| [9] | Krishna Ngoma Doumou (2008), "Dosage spectrophotométrique du permangate de potassium contenu dans les liqueurs de Dakin", Mémoire ENS de l’Université Marien NGOUABI. |
| [10] | N’GOKA Victor, Jean Grégoire OSSEBI (2014), "Etude qualitative des liqueurs de Dakin utilisées à Brazzaville", Eur. J. Sc. Research, 118(3°): 349- 357. |
| [11] | DURAND PRODUCTION, "Universel constructeur- pour Peugeot-Citroën". |
| [12] | Prestone (December 2016) "Engineered with cor-guard corrosion inhibitors prestone’s newest coolant is 5x more effective at protecting against corrosion". |
| [13] | TRIBOLOGIK Infolettre (2013), "L’analyse des liquides et huiles de coupe". |











 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML





