-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2020; 10(1): 1-5
doi:10.5923/j.chemistry.20201001.01

The Mechanism of Nitric Acid Degradation of the C-glycoside Aloin to Aloe-emodin
Francisco Sánchez-Viesca, Reina Gómez
Organic Chemistry Department, Faculty of Chemistry, National Autonomous University of Mexico, Mexico City (CDMX), Mexico
Correspondence to: Francisco Sánchez-Viesca, Organic Chemistry Department, Faculty of Chemistry, National Autonomous University of Mexico, Mexico City (CDMX), Mexico.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Although the majority of natural glycosides are O-glycosides, there are many examples of C- glycosides. Since in these glycosides the aglycone and the sugar are linked by a C-C bond they cannot be cleaved by acid hydrolysis. So, various biochemical procedures using enzymes or bacteria have been described for this purpose. However, these methods are troublesome and time consuming. A chemical degradation using nitric acid has been informed in a U.S. Patent, but there is no theoretical approach to this procedure. In this communication we provide a sustained reaction mechanism for this hydrolytic and oxidative degradation. The electron flow for each step is given and also an explanation of why simple hydrolysis cannot work, pointing out at which step the reaction comes to a stop.
Keywords: Aloe-emodin, Aloin, C-glycosides, Oxidative degradation, Reaction mechanisms, Reactive intermediates
Cite this paper: Francisco Sánchez-Viesca, Reina Gómez, The Mechanism of Nitric Acid Degradation of the C-glycoside Aloin to Aloe-emodin, American Journal of Chemistry, Vol. 10 No. 1, 2020, pp. 1-5. doi: 10.5923/j.chemistry.20201001.01.
1. Introduction
- Most of the plant glycosides are O-glycosides, however there are many examples of C-glycosides. The structures of fifteen naturally occurring C-glycosides are displayed in a recent article [1].Due to the firm C-C bond between sugar moieties and aglycones, C-glycosides cannot be deglycosylated by traditional hydrolysis.An alternative for this purpose is the use of biological chemistry. For instance, a bacterial type capable of deglycosylating some C-glycosides has been just reported [2].However, these methods are time consuming and imply a different scientific discipline.A chemical degradation of a C-glycoside has been described in a U.S. Patent [3]. Of course it deals only with the experimental procedure. It employs nitric acid and ethyleneglycol as solvent.In this communication we provide the reaction mechanism of this hydrolytic and oxidative cleavage, and explain also at what stage ordinary hydrolysis stops and why. This paper is a follow-up of our studies on chemical reaction mechanisms [4-7].
2. Antecedents
- Some reactions related with the matter of this paper are commented next.Nitric acid is a versatile acid. It can be part of the final product as in the reaction with polyalcohols to form organic-inorganic esters. These mixed esters are interesting due to their very different dual properties. For instance, glyceryltrinitrate (nitroglycerin) is the basic explosive of dynamite but this ester can be used for angina pectoris management [8,9].Aromatic nitration, a well known reaction, is enhanced by phenols.Other use of nitric acid is as oxidant in Carbohydrate Chemistry. The aldaric acids, α,ω-dicarboxylic acids, are obtained from open-chain sugars oxidized by nitric acid. Saccharic acid (glucaric acid) has been prepared this way [10]. The oxidation of selected alcohols and ketones by nitric acid has been informed [11]. More recently, the oxidation of aliphatic alcohols and diols to carboxylic acids by means of nitric acid has been carried out in Russia [12].There is an interesting communication on modifications in the nitric acid oxidation of D-glucose to D-glucaric acid [13].A third class of reactivity observed with nitric acid is oxidative degradation and this chemical deportment is important in the present study, as we will see.An example of this is the oxidative degradation of indigo blue to isatin, Figure 1, [14]. In that communication we gave the mechanism of this reaction.
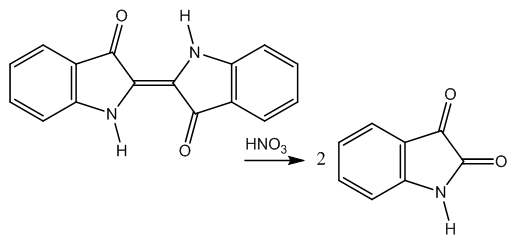 | Figure 1. Cleavage of indigo blue by nitric acid |
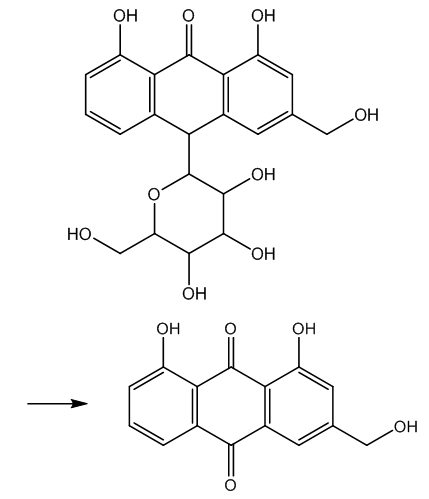 | Figure 2. Obtention of aloe-emodin from aloin |
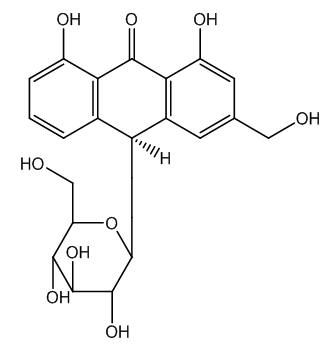 | Figure 3. Aloin A structure |
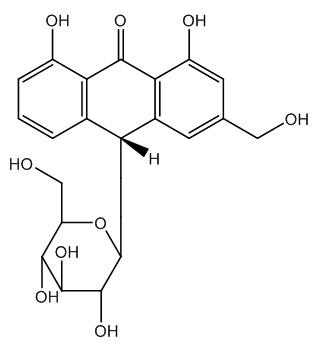 | Figure 4. Aloin B structure |
3. Discussion
- With aloin, none of the possible reactions with nitric acid took place, excepting degradative oxidation. This is due to different or especial reaction conditions. For instance, nitric ester preparation requires both concentrated and fuming nitric acids.In the case of aloin, a phenolic compound, aromatic nitration results interesting. Within the Patent reaction conditions there is not nuclear substitution, notwithstanding that it is known that the nitration of the phenols takes place readily [16]. This can be explained due to phenolic hydrogen bonding with the oxygen of anthraquinone. However, in a more drastic reaction aloetic acid is obtained, a tetranitro derivative [17-19], Figure 5.
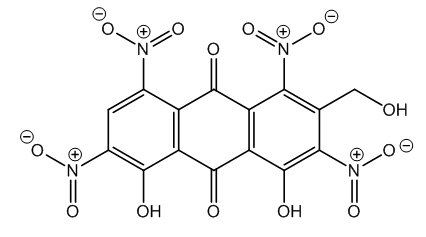 | Figure 5. 4,5-Dihydroxy-2-hydroxymethyl-1,3,6,8-tetranitroanthraquinone (Aloetic acid) |
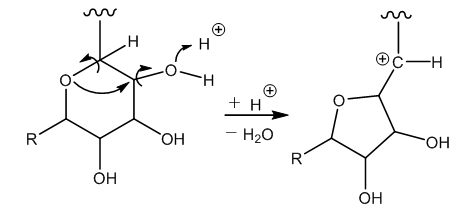 | Figure 6. Dehydration of the glucopyranose |
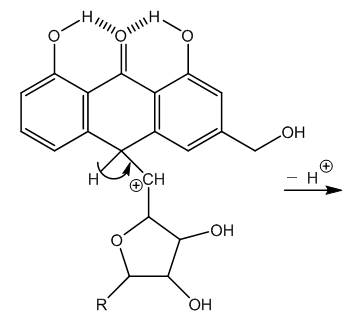 | Figure 7. Neutralization of the carbonium ion in absence of nitric acid |
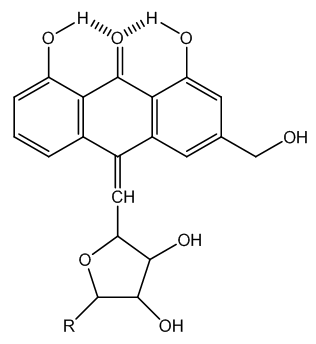 | Figure 8. Product that impedes C-glycoside fragmentation |
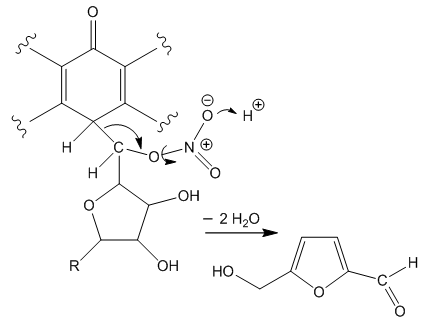 | Figure 9. Oxido-degradation of the C-glycoside |
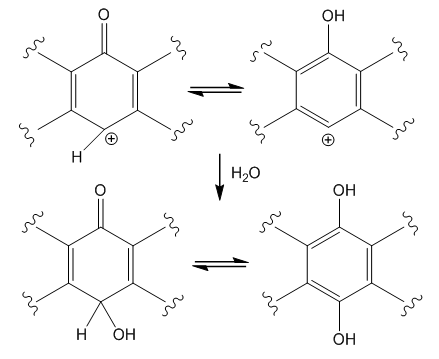 | Figure 10. Formation of an oxanthrone derivative |
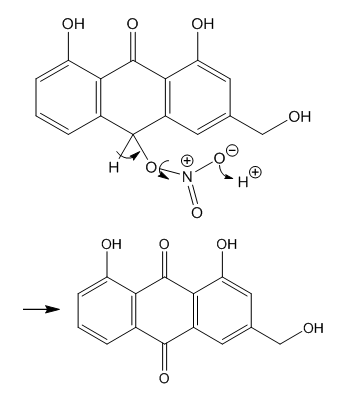 | Figure 11. Obtention of aloe-emodin by a second redox reaction |
4. Conclusions
- The O-glycosides can be hydrolysed but the C-glycosides are resistant to hydrolysis due to their C-C bond between aglycone and sugar. Since there are many naturally occurring C-glycosides, the obtention of these aglycones is very important because it simplifies considerably the chemical work for structural elucidation. Besides, they are preferable for conservation during storage.A U.S. Patent assigned to a Swiss Laboratory describes a method for the degradation of aloin, a C-glycoside, by means of nitric acid in ethlyleneglycol as solvent.Since there is no reaction mechanism for this oxidative degradation, we provide in this communication the pertinent theoretical explanation.The advanced mechanism is based on recent studies in Carbohydrate Chemistry. These results were applied to the pyranose structure in aloin, and the first part of the process was solved. This hydrolytic beginning yielded a key reactive intermediate.Then, there are two oxido reduction steps due to the action of the nitric acid. It is in the first one that the C-C fission occurs, the degradative oxidation. In the second redox reaction the anthraquinone derivative is obtained (aloe-emodin).Thus, the electron flow from aloin to aloe-emodin has been given step by step.An explanation of why simple hydrolysis doesn’t work has been supplied.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML