-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2019; 9(6): 159-164
doi:10.5923/j.chemistry.20190906.01

Complexes of Co(II), Cu(II) and Ni(II) with Antineoplastic Agent Imatinib Mesylate: Synthesis, Characterization and Biological Activity
Amira Cipurković1, Snježana Marić1, Emir Horozić2, Snježana Hodžić1, Darja Husejnagić1, Lamija Kolarević3, Amila Zukić3, Demir Bjelošević3
1Faculty of Natural Sciences and Mathematics, University of Tuzla, Tuzla, Bosnia and Herzegovina
2Faculty of Technology, University of Tuzla, Tuzla, Bosnia and Herzegovina
3Faculty of Pharmacy, University of Tuzla, Tuzla, Bosnia and Herzegovina
Correspondence to: Amira Cipurković, Faculty of Natural Sciences and Mathematics, University of Tuzla, Tuzla, Bosnia and Herzegovina.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of this work was to investigate the interaction of Imatinib mesylate with biological ions Cu(II), Co(II) and Ni(II) in ethanol/water solutions. Structures of synthesized complexes were characterized by spectroscopy methods. Stereo-microscopy was used for determination of morphological properties of obtained crystals. The results of IR spectroscopy showed that biogenic metal complexes with ImM were formed through the oxygen donors of mesylate ion. Changes of crystals colours and sizes of the parent ligand and complexes were clearly seen. Antimicrobial screening revealed a significant effect of Co(ImM)2 complex on the tested microorganisms. This complex also showed significant antioxidant activity compared to Ni(II) and Cu(II) complexes.
Keywords: Biometals, Complexes, Spectral characterization, FRAP, DPPH, Antimicrobial activity
Cite this paper: Amira Cipurković, Snježana Marić, Emir Horozić, Snježana Hodžić, Darja Husejnagić, Lamija Kolarević, Amila Zukić, Demir Bjelošević, Complexes of Co(II), Cu(II) and Ni(II) with Antineoplastic Agent Imatinib Mesylate: Synthesis, Characterization and Biological Activity, American Journal of Chemistry, Vol. 9 No. 6, 2019, pp. 159-164. doi: 10.5923/j.chemistry.20190906.01.
Article Outline
1. Introduction
- Biometal ions in biological systems usually interact with various parts of different organic and inorganic biomolecules via O-, N- and S-donor atoms. There are attraction between electron-rich functional groups in organic ligands and electron-deficient (cationic) metal ions, leading to a binding interaction between two species. The interaction of metal ions to suitable biological target binding site also depends on the valency and charge-accepting capacity of the metal ions. Recently, metal-based complexes have been extensively investigated. The products of interaction between M(II) metals and organic ligands (pharmaceutical substances) are fundamentally important for the coordination chemistry, as well as organic and medical chemistry.Imatinib (Im) is a competitive inhibitor of specific protein tyrosine kinases [1,2]. It inhibits intracellular ABL and BCR-ABL tyrosine kinases and tyrosine kinase receptors such as KIT and PDGFR [3,4]. First (imatinib) and second generation tyrosine kinase inhibitors, TKI (bosutinib, dasatinib, and nilotinib) represent effective, standard therapy for Philadelphia positive chronic myeloid leukemia (CML) [5,6]. Imatinib mesylate (ImM) exerts selective, dual inhibition of platelet-derived growth factor pathways, TGFβ, and PDGF, so imatinib exhibits antifibrotic effects on biologically relevant concentrations without toxic side effects, providing potential for its use in the treatment of fibrotic diseases systemic sclerosis [7]. Its safety and efficiencyfor patients with chronic myeloid leukemia and its efficiency as part of gastrointestinal stromal tumor treatment have been investigated in published papers [8-12]. ImM shows a high rate of cytogenic net and hematologic response, in cases where first-line drugs such as interferon are not effective [13,14]. ImMis currently recognized as a drug whose standard dosage shows excellent and long lasting responses in most patients with chronic myeloid leukemia, and as a drug that has revolutionized the treatment of patients with advanced and metastatic gastrointestinal stromal tumors, where it is one of the first choice because of its extreme efficiency [15-17]. However, resistance is one of the major problems in the treatment of patients with gastrointestinal stromal tumors. Several molecular mechanisms leading to ImM resistance have been reported, ranging from disorders in drug binding due to mutations, reduced efficacy due to amplification of the target gene, and reduced uptake of ImM into tumor cells due to recombinant MDR1/ABCB1 gene expression [18-22]. Preclinical basis, positive clinical experience, and high-dose ImM (600 mg) experience encourage the questioning of high doses of ImM as frontline therapy in patients diagnosed with CML or late-stage CML, to achieve better and more complete cytogenetic and molecular remissions [23,24]. In general, ImM therapy is well tolerated. However, adverse effects have been reported in clinical trials, most of which are mild or moderate in severity but also more serious such as gynecomastia, low testosterone, cytopenia, hepatotoxicity and cardiotoxicity [25-27]. ImM represents a significant progress in the development of cancer drugs, which will be able to be used in specific, targeted therapies for a number of malignancies, for which there are no sufficiently effective drugs [28]. The structure of imatinib mesylate is shown in Figure 1.
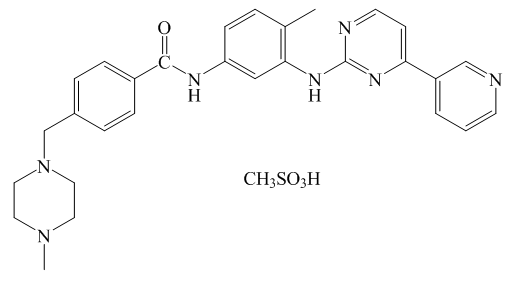 | Figure 1. Structure of imatinib mesylate |
2. Material and Methods
- An analytically pure sample of Imatinib mesylate was gifted from “ZADA Pharmaceuticals” company as an active substance. All the other chemicals (metal salts as hydrates and solvents) were of standard analytical grade and were used as supplied (Aldrich, Germany).
2.1. Synthesis of Complexes
- The synthesis of metal complexes was carried out according to the published method [29,30]. A mixture of ethanol and water, in a ratio of 50/50 (v/v), was used as a solvent for ligand and M(II) salts. Metals (M) and ligand (ImM) were mixed in a 1:2 molar ratio (n/n). The solutions of the appropriate metal (10 mL) and the ligand (10 mL) were mixed in a glass and stirred on a magnetic stirrer, without heating. The pH of the solutions was adjusted with 1 mol/L NaOH. The optimum pH value for model systems Co(II) -ImM and Ni(II)-ImM was 7.3 and for the model system Cu(II)-ImM the pH was adjusted to 5.6. Prepared solutions were mixed in a magnetic stirrer for half an hour and then left to stand in a dark place for two weeks in order to precipitate complexes. The resulting products were filtered and then dried at room temperature.
2.2. Spectral Characterization
- In order to determine the structure of the complex, the samples were recorded on a Nicolet iS10 FTIR Spectrophotometer - Thermo Fisher Scientific. The ATR technique was used for sample analysis. Samples were recorded in the range of 4000-650 cm-1.Due to the much better dissolution of the synthesized complexes and ligand in dimethyl formamide than in some other solvents, DMF was used as a solvent to prepare their solutions for UV spectral analysis. The solutions were recorded in the wavelength range of 200-400 nm.
2.3. Morphological Characterization
- Morphological analysis of solid samples allows differences in size, colour and shape to be observed. The colour, size and texture of the ligand and M(II) complex crystals were analyzed on a Leica DM 2500P binocular microscope. If crystals of pharmaceuticals exhibit polymorphism, their physical properties such as density, melting point, solubility and stability, bioavailability and processability will be dependent on the crystal form [31]. To determine the antimicrobial and antioxidant activity, solutions of ligand and M(II) complexes of concentration 5.0 mg/mL were prepared. DMSO was used as a solvent.
2.4. Antimicrobial Activity in vitro
- Antimicrobial activities were investigated by diffusion method for reference bacterial strains E.coli, E. faecalis, S. aureus, B. subtilis, L. monocytogenes and P. aeruginosa. Antifungal activity of the complex was tested on C. albicans. In the agar sterile drill-shaped holes were made ("wells") into which 80 μL of ImM and M(II) complex solutions of concentration 5.0 mg/mL were added. After the plates were left at room temperature for 15 min, the substance was diffused into agar, incubated at 37 °C/24 h.
2.5. Antioxidant Activity in vitro
- DPPH method. The percentage inhibition of DPPH radicals was examined according to a published method [32]. The volume of 0.5 mL of ligand solution or M(II) complex was transferred to a test tube and supplemented with methanol to 2.0 mL. After that 0.5 mL of 0.5 mM DPPH solution was added. The solutions were incubated for 30 minutes in the dark, then the absorbance at 517 nm was recorded. The volume of 0.5 mL 0.5 mM DPPH solution dissolved in 4.0 mL of methanol was a limit. The inhibition of DPPH radicals was calculated according to the equation:
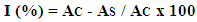 where: AC is the absorbance of the control and AS is the absorbance of the sample.FRAP method. To prepare the calibration curve, solutions of FeSO4 x 7H2O was prepared in the concentration range of 200-1000 mmol/L. The volume of 3.0 mL FRAP reagent was measured in five tubes and 0.1 mL standard solutions were added there too. The absorbance was measured with regard to a blank sample (3 mL FRAP reagent and 0.1 mL of water). In each tube, 0.2 mL of ligand or M(II) complexes (concentration 5.0 mg/mL) and 6.0 mL of FRAP reagent were added. The samples were incubated in an aqueous bath for 30 minutes at 37°C, and the absorbance was measured at 593 nm with regard to a blank sample (6.0 mL FRAP reagent and 0.2 mL DMSO) [33].
where: AC is the absorbance of the control and AS is the absorbance of the sample.FRAP method. To prepare the calibration curve, solutions of FeSO4 x 7H2O was prepared in the concentration range of 200-1000 mmol/L. The volume of 3.0 mL FRAP reagent was measured in five tubes and 0.1 mL standard solutions were added there too. The absorbance was measured with regard to a blank sample (3 mL FRAP reagent and 0.1 mL of water). In each tube, 0.2 mL of ligand or M(II) complexes (concentration 5.0 mg/mL) and 6.0 mL of FRAP reagent were added. The samples were incubated in an aqueous bath for 30 minutes at 37°C, and the absorbance was measured at 593 nm with regard to a blank sample (6.0 mL FRAP reagent and 0.2 mL DMSO) [33].3. Results and Discussion
3.1. Structure of Complexes
- Based on the FTIR spectra, it is assumed that Imatinib mesylate coordinates metal ions as a bidentate O-donor ligand in the molar ratio M:L=1:2. The mesylate part of ImM molecule is involved in bond formation, that is, two S=O groups of each mesylate. It is possible to form a complex of square-planar structure or octahedral complex with two water molecules in axial positions. A stoichiometry for all the synthesized ligand-metal complexes should be characterized by elemental analyses. The proposed structure of obtained complexes is shown in Figure 2.
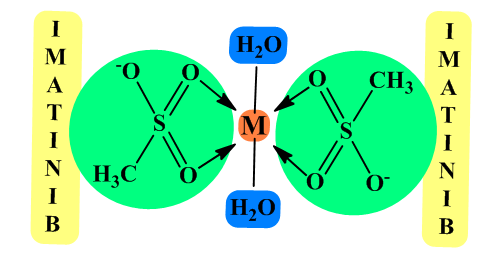 | Figure 2. Proposed structure of the M(II) complexes with ImM |
3.2. Spectral Characterization
- The IR spectra of the complexes were compared with that of the free ligand to determine the changes that might have taken place during the complexation. The results obtained correspond well with literature data [34]. The ATR-FTIR spectrum of ImM showed characteristic bands at 3255 cm-1 (N-H stretching vibration), 1658 cm-1 (C=O band), 1570, 1525, and 1444 cm-1 (aromatic C=C, C=N stretching vibration), 1159 cm-1 (C-N stretching vibration), 1036 cm-1 (C-O stretching vibration), and 807 cm-1 (aromatic C-H deformations out of plane). In all spectra, complexes of ImM with metal ions results in a pronounced decrease in the intensity of the bands in the regions 1060-1020 cm-1 S=O stretching (1036 cm-1), 1190-1120 cm-1 SO2 symmetric stretching (1159,67 cm-1), indicating possible interactions of Co(II) ions with O- or S- donor atoms of mesylate ion. The UV spectrum of the parent ligand in DMF is characterized by an absorption maximum at 268 nm, which is approximately the literary data for the same compound dissolved in distilled water, on 255 nm [35], 256 nm [36] and 281 nm [37]. The absorption maximum according to the literature indicates that these are
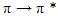 transitions, due to the presence of unsaturated bonds and heteroatoms in the Imatinib mesylate molecule. The synthesized complexes have an absorption maximum at the same wavelength as the parent ligand, but with higher absorbance values. Values of absorbance increase in the following order: Cu(ImM)2 > Ni(ImM)2 > Co(ImM)2.
transitions, due to the presence of unsaturated bonds and heteroatoms in the Imatinib mesylate molecule. The synthesized complexes have an absorption maximum at the same wavelength as the parent ligand, but with higher absorbance values. Values of absorbance increase in the following order: Cu(ImM)2 > Ni(ImM)2 > Co(ImM)2.3.3. Morphological Characterization
- Figure 3. shows images of ligands and M(II) complexes taken on a binocular microscope. Nicholas's prisms were placed in a vertical position (XPL) when microphotographing. For XPL nickels, the interference colours for ImM are gray and pale yellow-brown. When turning off Nicholas (PPL), no colours appeared in the sample. The crystals are small (0.1-0.3 mm), rarely larger (0.5 mm). The crystals are irregular in shape-anhedral. In addition to the size and shape, radial-ray material was observed for crystals concentrated in the central part of the image (yellow-brown colour). The crystals in the marginal parts of the image (gray interferon colour) have two lines of graft that are almost perpendicular to each other. The crystals of Cu(II) complex interfere with first-order vibrant colours. The interference is expressed along the base of the crystal. The crystal size is wide (up to a maximum of 0.1 mm), with a mean value of 0.04 mm. The crystals appear in rhombohedral forms, with one elongated axis. The crystals of Co(II) complex interfere with first-order vibrant colours. The interference is expressed along the edges of the crystals. The crystal size is wide (up to max. 0.2 mm), with a mean value of 0.06 mm. The crystals appear in rhombohedral forms, with one elongated axis. The crystals of Ni(II) complex interfere with first-order vibrant colours. The crystal size is wide (up to max. 0.1 mm), mean 0.05 mm. The crystals differ in morphology. In Figure D1 the crystals are an oval-elliptic shape. Radial-air aggregates (center-to-edge growth) are characteristic. In Picture D2, observed crystals are in an elongated prismatic shape- sized 0.12 mm.
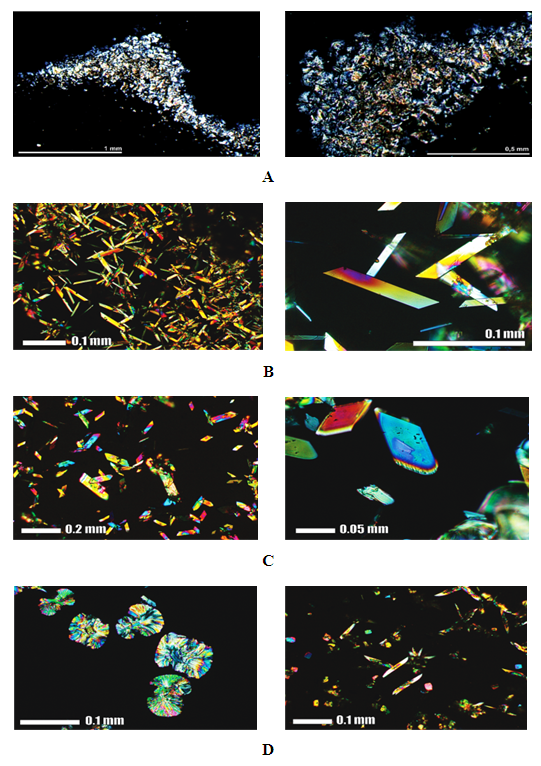 | Figure 3. Crystal morphology: (A) Imatinib mesylate; (B) Cu(II) complex; (C) Co(II) complex and (D) Ni(II) complex |
3.4. In vitro Antimicrobial Activity
- The ligand, as expected, showed no antimicrobial activity on the tested microbial strains at a concentration of 5 mg/mL. A complete absence of antimicrobial activity was also observed in the Cu(ImM)2 and Ni(ImM)2 complexes. However, the Co(ImM)2 complex had a significant zone of inhibition in certain bacterial strains and C. albicans. The range of 16-20 mm inhibition zones was recorded in B. subtilis, L. monocytogenes and S. aureus, while the inhibition zone for P. aeruginosa and C. albicans was 14 and 15 mm, respectively. Although the inhibition zones are higher than expected, these complexes cannot be considered effective antimicrobial agents because the tested concentration is extremely high (5.0 mg/mL) as opposed to the positive control (Ciprofloxacin, concentration 1 mg/mL) much larger inhibition zones (>30 mm).
3.5. In vitro Antioxidant Activity
- The FRAP method reduced the Co(ImM)2 complex at 5.0 mg/mL to 770.2 μmol/L Fe2+. For other compounds, no reductive ability was detected at the indicated concentration. Provided FRAP value for Co(ImM)2 complex was significantly lower compared to the positive control (vitamin C, conc. 1.0 mg/ml) which FRAP value is 14 250 μmol/L of Fe2+. Therefore, cobalt compound can be classified as having less antioxidant capacity. Figure 4. shows a graph of DPPH radical inhibition by solutions of ligands and M(II) complexes.
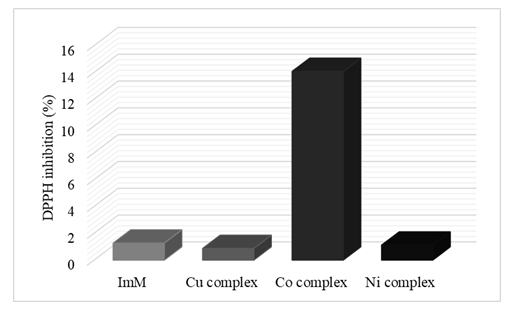 | Figure 4. Graphic representation of DPPH radical inhibition |
4. Conclusions
- Spectral and morphological analysis revealed the interaction of Imatinib mesylate with biogenic metals Cu, Co and Ni, forming complexes of the stoichiometric ratio ML2. The coordination bond between metal ion and the ligand is achieved through the oxygen donor atoms of the S=O group of mesylate ions. The complexes are in various colours and shapes different from the colour and shape of the ligand indicating that the colour and shape formed depend on the transition metal ions. Antimicrobial screening revealed a significant effect of Co(ImM)2 complex on the tested microorganisms. This complex also showed significant antioxidant activity compared to Ni(II) and Cu(II) complexes.
ACKNOWLEDGEMENTS
- This work was financially supported by grant 05-39-2518-1/18 from the Federal Ministry of Education and Science, Bosnia and Herzegovina in 2018.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML