-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2019; 9(4): 123-126
doi:10.5923/j.chemistry.20190904.03

On the Mechanism of the Neumann-Wender Glucose Test
Francisco Sánchez-Viesca, Reina Gómez
Organic Chemistry Department, Faculty of Chemistry, National Autonomous University of Mexico, Mexico City (CDMX), Mexico
Correspondence to: Francisco Sánchez-Viesca, Organic Chemistry Department, Faculty of Chemistry, National Autonomous University of Mexico, Mexico City (CDMX), Mexico.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This sensitive test for glucose in urine is based on the discolouration of a methylene blue solution by reaction with glucose in alkaline medium. This reaction, combined with aerial oxidation to restore the blue colour has been widely used as a showy experiment. However, the mechanism of the involved reactions has not been advanced and we provide it in this communication. The glucose enolate combines with methylene blue and then an oxido-reduction takes place by a concerted mechanism. The last reaction is a Friedemann cleavage of an alpha-ketoaldehyde into acids by means of hydrogen peroxide in alkaline medium. The mechanism of this fragmentation has been also cleared up.
Keywords: Azanium, Degradation, Methylene blue, Oxido-reduction, Quinone-di-imine, Resonance, Thiazinium
Cite this paper: Francisco Sánchez-Viesca, Reina Gómez, On the Mechanism of the Neumann-Wender Glucose Test, American Journal of Chemistry, Vol. 9 No. 4, 2019, pp. 123-126. doi: 10.5923/j.chemistry.20190904.03.
1. Introduction
- Methylene blue is a dye used in optical microscopy to stain nuclei of animal tissues. It is suitable as a bacterial stain and also as vital stain, i.e., can be applied on living cells without killing them [1].The frame structure of methylene blue is dibenzo[b,e] [1,4] thiazine, [2]. The numbering is indicated [3,4]. Other names are thiodiphenylamine and phenothiazine. Two structures have been advanced for methylene blue [5], Figure 1. These structures will be discussed afterwards.
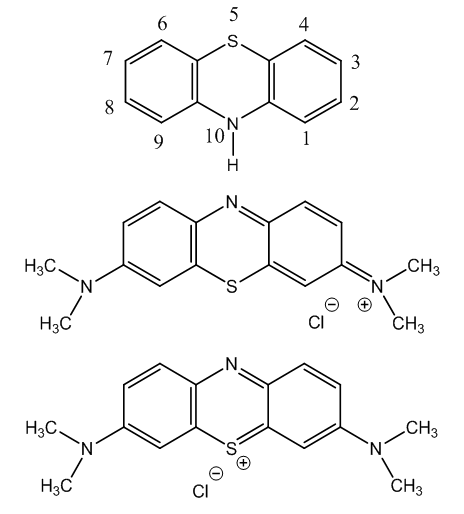 | Figure 1. Azanium chloride and phenothiazinium chloride structures for methylene blue |
2. Antecedents
- There is a brief note entitled “Methylene blue-Reduction and Oxidation”, with no formulas or references. It is only a rapid demonstration without comment or explanation, only to stimulate interest, [14].Campbell published an article on kinetics and it is a puzzle for students in order to discover the presence of a gas in a closed flask that can restore the blue colour on shaking. No product is mentioned [15].Other article is “Blue Bottle Experiment: Learning Chemistry without knowing the Chemicals”. It is indicated for First Year Undergraduate and General Public, a popular chemical demonstration “because of its simplicity and visual appeal” [16].As it can be seen, these are colourful presentations.There is a communication of Anderson et al. [17] with a confusing title: “The Methylene Blue-Catalysed Air Oxidation of Glucose”. It is leukomethylene blue which is oxidized by oxygen in the alkaline medium. Although the reactants, the catalyst and reaction products are mentioned and their structures are given, there is no reaction mechanism at all. For instance, the reduction of methylene blue to the colourless compound is indicated by:
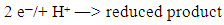 There are no free electrons in the reaction medium, stripped away from the atoms, and even less protons in an alkaline medium. The authors have forgotten the collision theory, that is, close contacts among reactive species.In these articles the Neumann-Wender test for glucose is not mentioned at all, despite its application in Medicinal Chemistry as a qualitative analytical method. In this communication this omission has been repaired and the mechanism of the involved reactions is provided.
There are no free electrons in the reaction medium, stripped away from the atoms, and even less protons in an alkaline medium. The authors have forgotten the collision theory, that is, close contacts among reactive species.In these articles the Neumann-Wender test for glucose is not mentioned at all, despite its application in Medicinal Chemistry as a qualitative analytical method. In this communication this omission has been repaired and the mechanism of the involved reactions is provided.3. Discussion
- As said before, two structures have been advanced for methylene blue with their respective names. But what structure represents better the chemical deportment of the compound? The structure with the azanium cation presents a quinone-di-imine group, an indamine [18].The other structure, with a thiazinium, presents an o-quinone type structure. In this representation the positive charge is in the sulphur which is less electronegative (2.5) than nitrogen (3.0), [19]. Besides, this structure presents an electrophilic centre at the alpha position to the thiazinium atom, whereas the other structure does not present a reactive centre.The structures are electromeric [20], as is shown in Figure 2.
 | Figure 2. Methylene blue electromeric structures |
 | Figure 3. Redox reaction of glucose with methylene blue by a concerted mechanism |
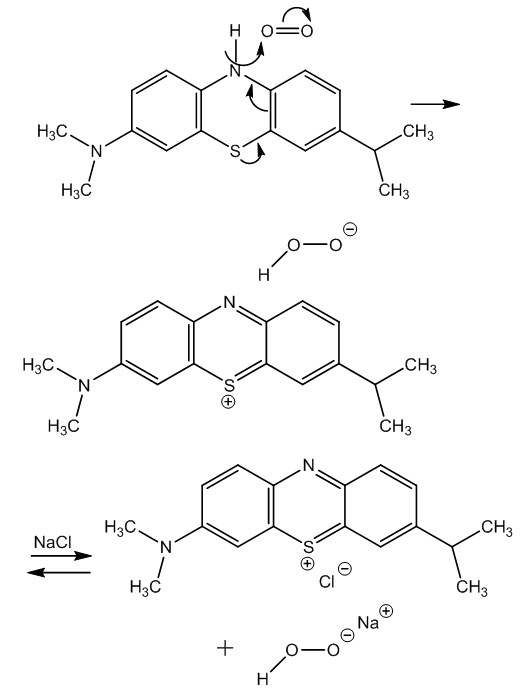 | Figure 4. Mechanism of aerial oxidation of leukomethylene blue in alkaline medium |
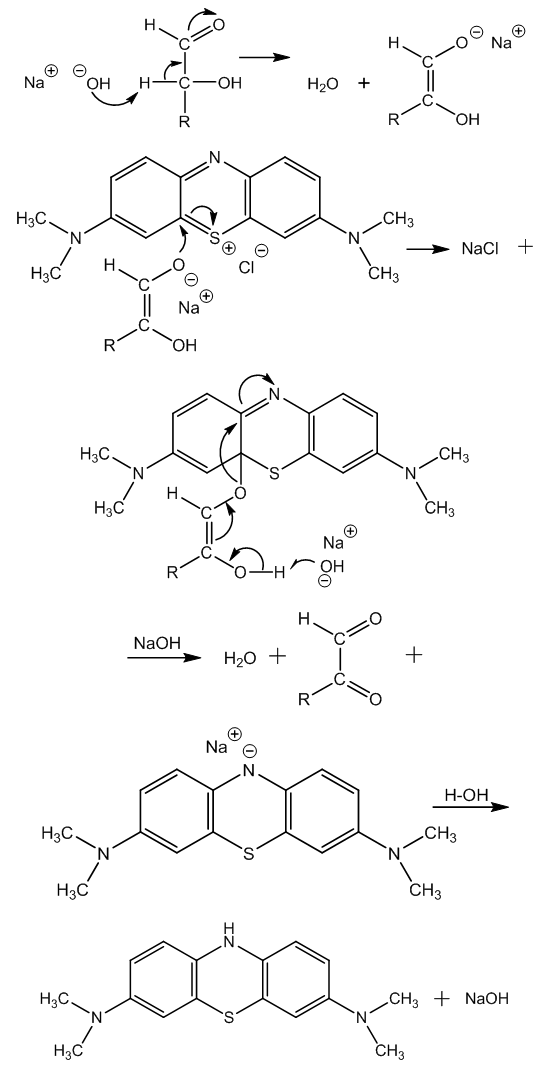
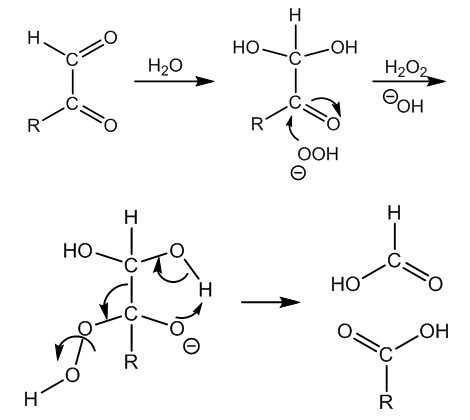 | Figure 5. Mechanism of the Friedemann cleavage of α-ketoaldehydes by means of hydrogen peroxide in alkaline medium |
4. Conclusions
- The two advanced structures of methylene blue are discussed in order to choice the structure that represents better the chemical deportment of the compound.The articles related to the oxido-reduction reaction between glucose and methylene blue in alkaline medium, followed by aerial oxidation, are commented.We provide the mechanism for these reactions since there is none. The glucose enolate combines with methylene blue (adduct formation) and the oxido-reduction step takes place by a concerted mechanism of push-pull type.The aerial oxidation of leukomethylene blue in alkaline solution occurs by a synchronous electron shift from an electron donor atom to molecular oxygen as hydride scavenger, through an intermediate double bond.Thus, the Neumann-Wender reaction mechanism has been cleared up.The mechanism of the Friedemann cleavage of α-ketoaldehydes into acids by hydrogen peroxide and KOH has also been provided (a concerted reaction mechanism). The driving force is the push-pull effect from an alkoxide to the δ+ oxygen at a hydroperoxide chain, involving a C―C break.
ACKNOWLEDGEMENTS
- Thanks to co-worker Martha Berros for useful dialogue.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML