-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2019; 9(4): 115-122
doi:10.5923/j.chemistry.20190904.02

Catalytic Activation and Deactivation of Pt-Pd Film Immobilized on Nafion Surface in Attaining Decomposition of Hydrogen Peroxide
M. M. Alam 1, 2, M. Humayun Kabir 1, 3, M. Ahsan 1, S. M. Borhan Uddin 1, I. A. Siddiquey 1, M. A. Hasnat 1
1Department of Chemistry, Shahjalal University of Science and Technology, Sylhet, Bangladesh
2Department of Arts and Sciences, Bangladesh Army University of Science and Technology (BAUST), Saidpur Cantonment, Nilphamari, Bangladesh
3Department of Chemistry, Noorjahan Memorial Women’s Degree College, National University, Bangladesh
Correspondence to: M. A. Hasnat , Department of Chemistry, Shahjalal University of Science and Technology, Sylhet, Bangladesh.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Bi-metallic Pt-Pd catalytic films were immobilized on Nafion-117 membrane to decompose hydrogen peroxide. A basic medium helps to enhance autocatalysis of hydrogen peroxide decomposition reaction. Although H2O2 decomposition is strongly increased by the presence of iodide ions and sodium dodecyl sulfate species but they deactivate the catalytic surface. Among several sulfate salts (CoSO4.7H2O, K2SO4 and CuSO4), only NiSO4.7H2O influenced to increase the reaction rate. Meanwhile, the Hg2+ species not only inhibits the decomposition process but also permanently damage the activity of the catalytic surface.
Keywords: Pt-Pd catalyst, Electrolytes, Decomposition, Hydrogen peroxide, Nafion membrane
Cite this paper: M. M. Alam , M. Humayun Kabir , M. Ahsan , S. M. Borhan Uddin , I. A. Siddiquey , M. A. Hasnat , Catalytic Activation and Deactivation of Pt-Pd Film Immobilized on Nafion Surface in Attaining Decomposition of Hydrogen Peroxide, American Journal of Chemistry, Vol. 9 No. 4, 2019, pp. 115-122. doi: 10.5923/j.chemistry.20190904.02.
Article Outline
1. Introduction
- The structural formula of Hydrogen Peroxide is H-O-O-H. It contains O-O bond which is responsible for destructive decomposition of hydrogen peroxides. It is an environmentally safe reagent and a very important intermediate in various environmental and biological reactions. It is widely used in the pulp and paper industry, mining, cosmetic and pharmaceutical industries, as well as common household cleaners such as disinfectants, antiseptics, bleaching and decolorizing agents [1,2]. It is used as the source oxygen in fuel cells [3]. Due to chemical structure and unpaired electrons, H2O2 is a well-known strong oxidant [4] for not only inorganic materials, such as carbon nanomaterials, metals, and semiconductors, but also organic ones including biomolecules. The most important factor of this green chemical is that it produces only water and oxygen through the oxidation process [5]. In biological aerobic organisms, H2O2 is better recognized for its toxic effect inside living cells. Various biochemical reactions involving oxidase enzymes, like alcohol oxidase, urate oxidase, amino acid oxidase, cholesterol oxidase, glucose oxidase, generate H2O2 as byproduct [6]. H2O2 is a well-established source of hydroxyl radical (∙OH) in the so-called oxidative destruction of organic wastes [7-9]. This radical causes different kinds of disorder in the body such as Alzheimer’s, myocardial infaction, atherosclerosis, Parkinson’s, diabetes, ageing, cancer, etc. [10-13]. The peroxo-oxygen of H2O2 is very unstable and easily undertakes disproportion reaction hence widely used in home-made small explosions. Several incidents have been reported where peroxide based small explosives were used in terror attacks [14–17]. Hence, studies on H2O2 decomposition are important in both chemistry and biochemistry.Many efforts have been employed to destroy toxic organic compounds using H2O2 in presence of UV and visible light [18-21]. A few metal oxide catalysts are identified as effective decomposition agents of diluted hydrogen peroxide. A very important and practical catalyst for diluted H2O2 decomposition is MnO2 [22]. Other examples of H2O2 decomposition catalysts are iron-cobalt oxides [23], certain rare earth perovskites [24], copper (II) ions [25], water-ceramic interfaces [26], activated carbon [27], etc. Earlier in a report, we have discussed the role of Fenton’s reagent (Fe(II)/Fe(III) + H2O2), for attaining rapid degradation of some organic compounds [28]. Although nearly all the H2O2 was decomposed during the degradation process, it was necessary to remove the residual peroxide as it is to avoid the secondary pollution. If the waste containing Fenton’s reagent is discharged to the natural aquatic systems, it may cause harm to the living beings to the action of ∙OH radicals. By contrast, when analytical samples containing such oxidative materials are employed into sophisticated techniques like chromatography, the column materials might be degraded or damaged. Therefore, prior to inserting the test samples through the column, it is necessary to remove all the residual hydrogen peroxide.In case of the decomposition of H2O2 by catalyzing different metal oxides, due to chemical reactions, metal oxides are also consumed proportionally to the extent of hydrogen peroxide. As the metal oxides cannot be generated spontaneously, therefore further action is required to avoid the secondary pollution caused by alkaline earth or transition metal ions. Conversely, noble metals seem to show pitiable surface catalytic performance for numerous decomposition reactions. A highly selective catalyst capable to decompose H2O2 is Pt sol with a rate constant of 7.1 × 102 s-1 [29]. Jones reported that palladium is even more effective than platinum [30]. Moreover, bi-metallic catalysts, originated from Pt and Pd have been fascinated by the researchers due to their robust catalytic properties for various reactions [31-33]. Recently, we have shown how bimetallic catalyst decomposes hydrogen peroxide [34]. Pt and or Pd adatoms assembled on Nafion–117 membrane is easy to prepare, stable and give large geometric area, i.e., large number of catalytic sites for executing different catalytic reactions [6,9,10]. In the previous studies, we have shown how noble metal films fabricated on membrane pores efficiently catalyzes hydrogen peroxide decomposition as displayed in scheme 1 [6,9]. Several researchers have been reported that the decomposition activity of hydrogen peroxide is found to decrease drastically due to the addition of different halide anions (Cl-, Br- or I-) or in the acidic reaction medium in presence of Pd/C and other supported catalyst [35-37]. In our foregoing paper, we have shown how some alien materials such concentrations of acid, halides, oxides and sulphates, surfactant (SDS) etc. either activates or deactivates the catalytic activity of Pt-Pd/Nafion surface pertaining to decomposition of H2O2 at room temperature.
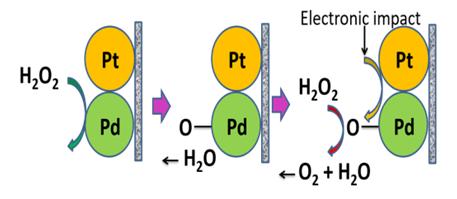 | Scheme 1. Hydrogen peroxide decomposition at Pt-Pd surface |
2. Experimental Design
2.1. Materials and Methods
- All the chemicals (salts, acids etc.) used were analytical grade, and were used without further purification which were collected from Merck, Germany and Nafion membrane was purchased from Walko Icorp, Japan. The preparation, characterization and catalytic efficiency of bimetallic Pt-Pd Nafion assembly were described in details in our earlier paper [36]. Pt and Pd particles were deposited on Nafion-117 membrane (DuPont Inc.) by chemically. Nafion membrane was first send blasted and dried at 110°C. This membrane is then immersed in a 250 mL pyrex glass made sample vial containing 200 mL water and 5 mg of H2[PtCl6]. A mixed solution containing 2.0 M NaBH4 and 4.0 M NaOH was poured in membrane containing solution at the rate of 20 mL h–1. At the meantime, temperature was raised from 35°C with 5°C h–1 increase for 12 hours. Pt particles were deposited on to Nafion surface with an area of 2 × 3 cm2 per side. By the same way, Pd particles were immobilized on Pt-covered Nafion surface by using 10 mg of PdCl2, which was collected from Merck, Germany. In this paper, the decomposition efficiency of H2O2 was studied with respect to time in presence of different halides, surfactant and different salts at room temperature. During decomposition, the influence of light was averted by wrapping the reactor with aluminum foil. A graduated glass burette was used to measure the volume of O2 at standard temperature (300K) and pressure (1.0 atm). The number of oxygen molecules were then evaluated using universal gas laws (i.e., PV= nRT). The decomposition of hydrogen peroxide was also determined quantitatively Iodometric titration method to measure the dissolved oxygen before and after reaction. The catalytic competence of Pt–Pd film deposited on Nafion–117 membrane was measured with respect to H2O2 decomposition. The concentration of H2O2 was adjusted to 1.0 M in all cases. The pH of the H2O2 solution was adjusted to the desired value by using dilute solutions of HCl or NaOH. After the reaction, the catalyst was washed with deionized water and dried and then characterized by SEM image and XPS technique. The morphology of Pt-Pd/Nafion was examined by FE-SEM (JEOL, JSM-7600F, Japan). Elemental analysis was investigated using EDS from JEOL, Japan. The XPS measurements were examined for the calculation of binding energies in eV of Pt and Pd on a MgKα1 spectrometer (Thermo scientific, Kα1066) with an excitation radiation source AlKα1, Beam spot size = 300.0 μm, pass energy = 200.0 eV,
 torr). The XPS scan was performed unless smooth curves were obtained.
torr). The XPS scan was performed unless smooth curves were obtained. 3. Results and Discussion
3.1. Influence of pH
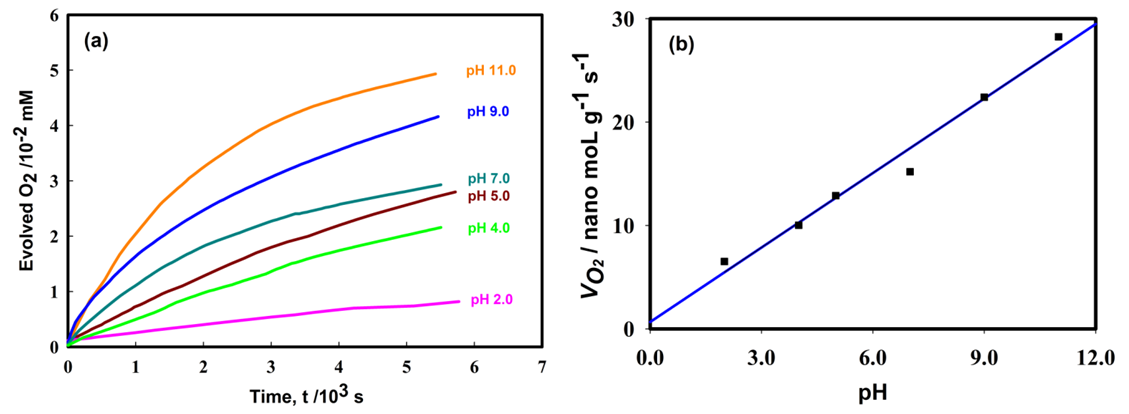
- Figure 1 shows the influence of solution pH on decomposition of hydrogen peroxide. It can be seen from the plot that the decomposition rate of H2O2 increased with the increase of solution pH and the evolution of oxygen occurred exponentially, where, after a certain time, evolution of oxygen reached to plateau in each experiment. The reason of enhanced decomposition rate could be explained by the decomposition mechanism and properties of the bi–metallic Pt–Pd surfaces. From decomposition mechanism, it can be seen that Pt–(OH) and Pt–(H) are directly involved in its decomposition kinetics. As the pH of the system increases, the number of negatively charged sites also increases. Negatively charged sites on bimetallic Pt-Pd favor the decomposition of hydrogen peroxide. Also, at lower pH, the H+ ion concentration in the system was increased and bi–metallic Pt–Pd surfaces perhaps acquired positive charges by absorbing H+ ions to form H3O+. Therefore, reformed electronic state of the surface probably inhibited the adsorption of H2O2 molecules on to the catalytic surface which declined the decomposition rate under acidic condition. Conversely, the favorable environment of adsorption was generated while the pH of the medium was increased that helped the autocatalysis of hydrogen peroxide decomposition. Figure 1(b) portrays that oxygen evolution rate shifted positively with increasing pH which follows a linear regression approximation, within the pH range 2 to 11 as per equation (1).
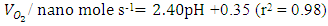 | (1) |
3.2. Influence of the Presence of Different Halides
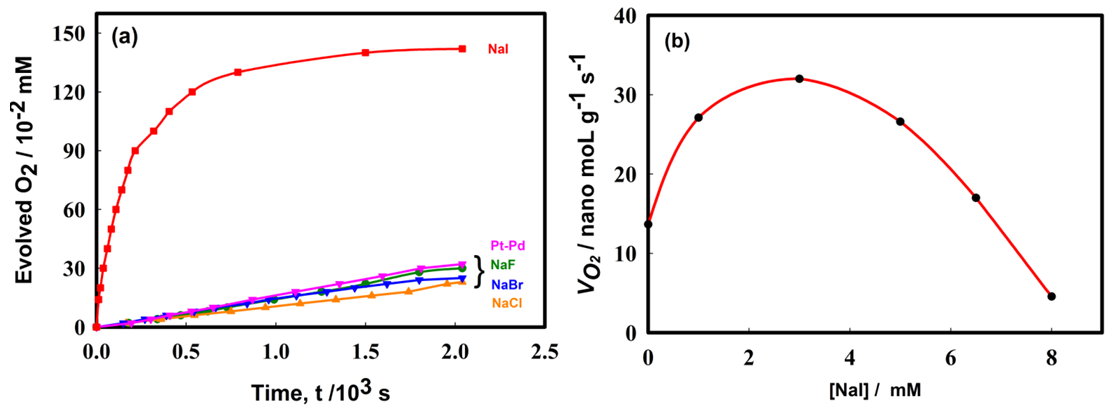
- Figure 2 shows the influence of various sodium halide salts as electrolyte in the reaction medium on hydrogen peroxide decomposition. It is interesting to note from the reported results that only NaI increased the H2O2 destruction rate, while NaF, NaBr and NaCl slightly influenced H2O2 decomposition process. The influence of halide ions on the rate of decomposition of H2O2 followed an order: I- >>>Br- >Cl->F-. In order to clarify the role of NaI, further decomposition of H2O2 was tested by varying the concentration of NaI from 0 to 8.0 mM. The result shows that increasing concentration of NaI increased the decomposition rate but above 3.7 mM of NaI concentration, the decomposition rate declined.
 | (2) |
 | (3) |
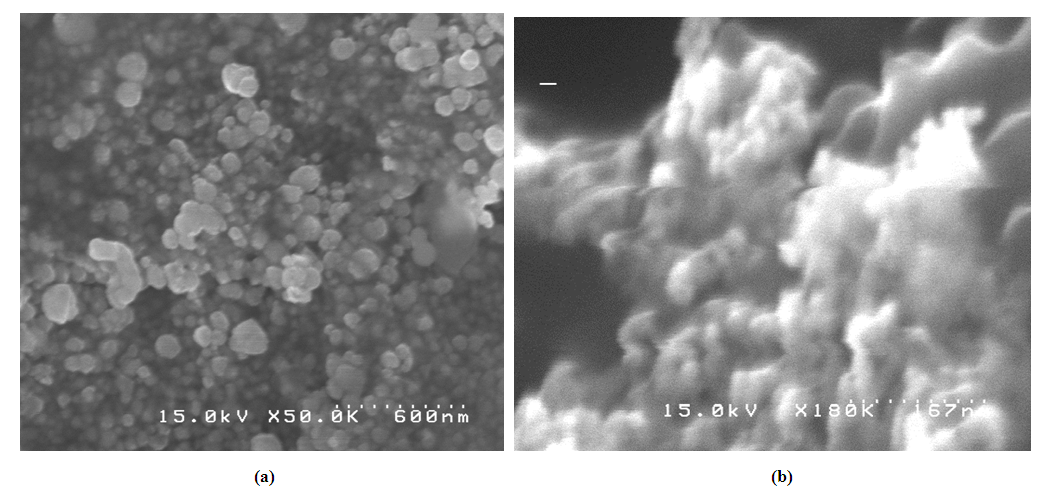 | Figure 3. SEM images of Pt-Pd/Nafion catalyst (a) before and (b) after using NaI. Other conditions are same as mentioned in Fig. 2 |
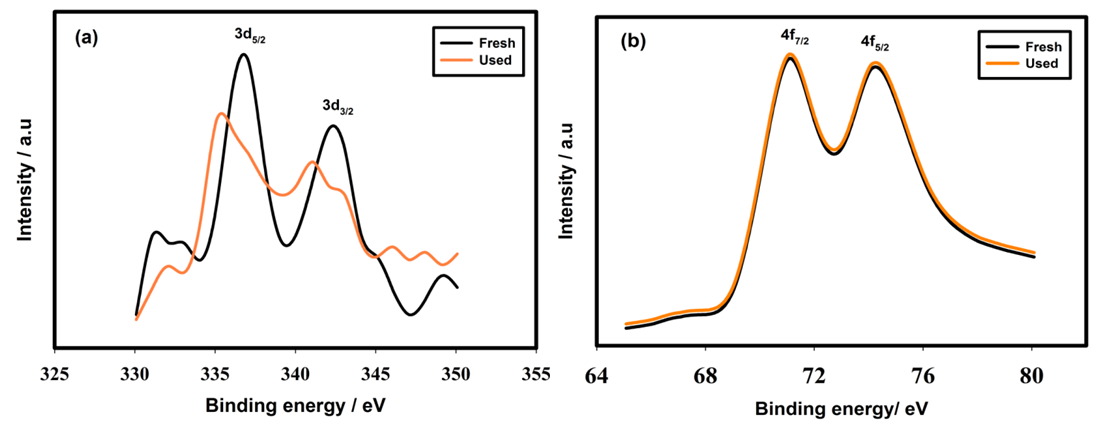 | Figure 4. XP spectra of Pd (a) and Pt (b) particles before and after use in presence of NaI. Other conditions are same as mentioned in Fig. 2 |
3.3. Influence of SDS
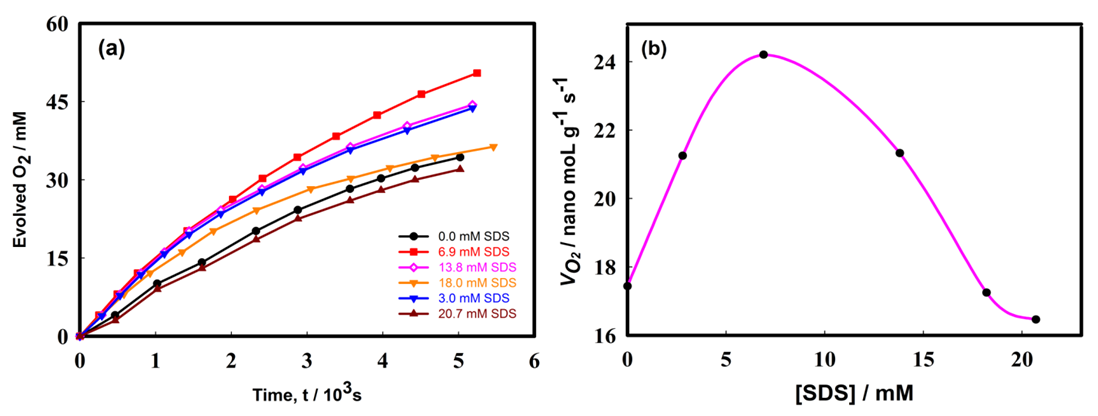
- Figure 5a shows the effect of concentration of sodium dodecyl sulfate (SDS) on the rate of H2O2 decomposition reaction which was carried out using a Pt-Pd/Nafion catalyst. From Fig.5b., it can be seen that the rate of hydrogen peroxide decomposition increased with increasing of SDS concentration within the concentration range of 0 to 6.9 mM. This phenomenon may be explained by the fact that SDS molecules have some cleansing effect which can efficiently remove organic residue/impurities from a surface. Thus, as the concentration of SDS was increased, the Pt-Pd surface increased its active sites until its concentration reached to 6.9 mM which is reflected by the increase of decomposition rate. However, above this threshold concentration of SDS, the hydrogen peroxide decomposition rate declined with the further increase of SDS concentration. At SDS concentrations higher than 6.9 mM, probably diffusion of H2O2 was retarded due to the increased population of SDS molecules and or Pt-Pd surface was poisoned by this surfactant which resulted decrease of H2O2 decomposition. To understand the fact of retardation of reaction rate, the morphology of Pt-Pd surface was examined after use. Figure 6 shows the SEM image of Nafion-supported Pt–Pd catalyst after the reaction being carried out in presence of SDS molecules. In comparison with fresh surface (shown in Fig. 3a), it is distinct that after contact with SDS, the morphology of the catalytic surface was significantly altered. Notably corrosion of the metallic faces on the surface is clearly visible from SEM image. Meanwhile, the surface’s activities could not be regenerated even when the experiment was re-conducted in absence of SDS molecules. Therefore, it can be concluded that SDS molecules poisoned or removed the active sites of the catalyst surface by declining decomposition rate.
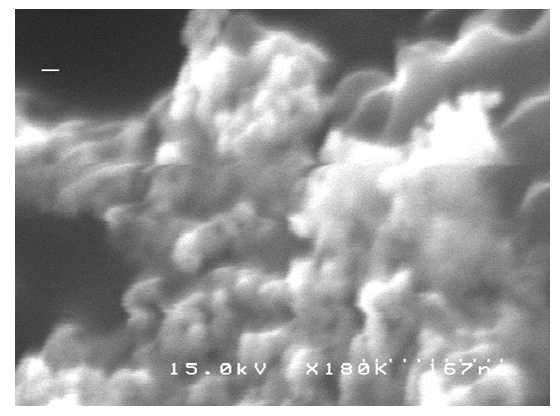 | Figure 6. SEM image of Pt-Pd/Nafion catalyst after reaction in presence of SDS |
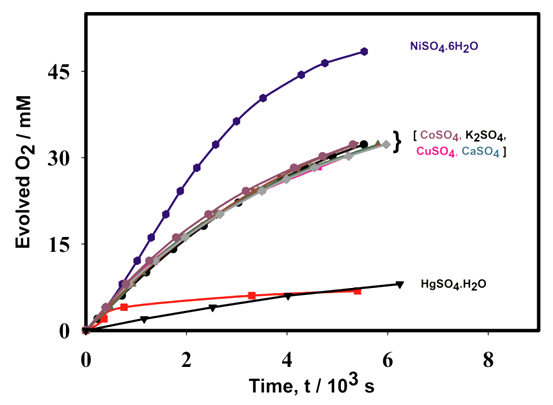
3.4. Effect of Various Metal Oxides and Sulfates
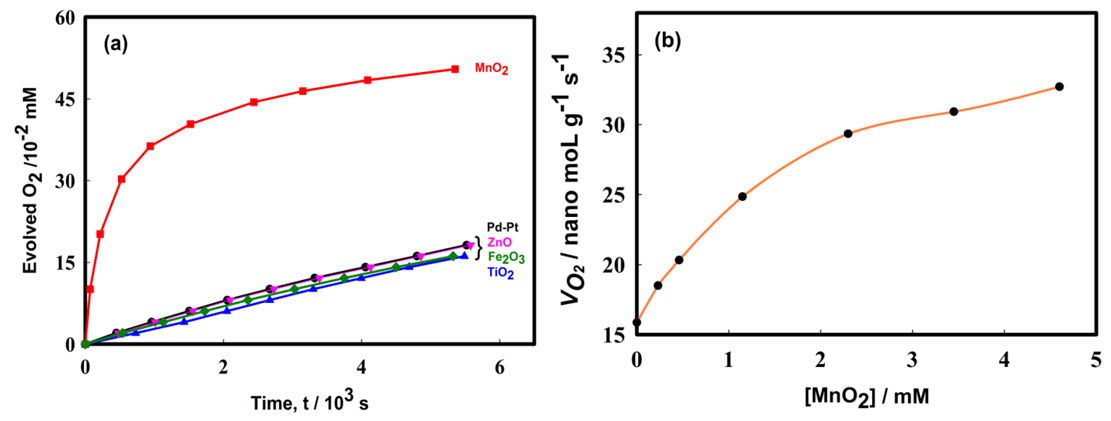
- Figure 7(a) shows the effect of various metal oxides on decomposition of H2O2 using the Pt-Pd catalytic surface. From this Figure, it is noticeable that only MnO2 increased the evolution rate of O2 considerably where, TiO2, ZnO, and Fe2O3 did not to show any substantial effect on decomposition of H2O2. This happened because MnO2 also decomposed H2O2 in parallel with the Pt-Pd surface. But the other metal oxides did not show similar function. Thus, in accordance with literature [39], the following reaction pathway may be proposed which took place simultaneously with decomposition of hydrogen peroxide on Pt-Pd surface:
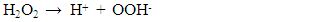 | (4) |
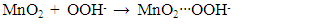 | (5) |
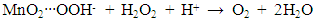 | (6) |
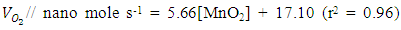 | (7) |
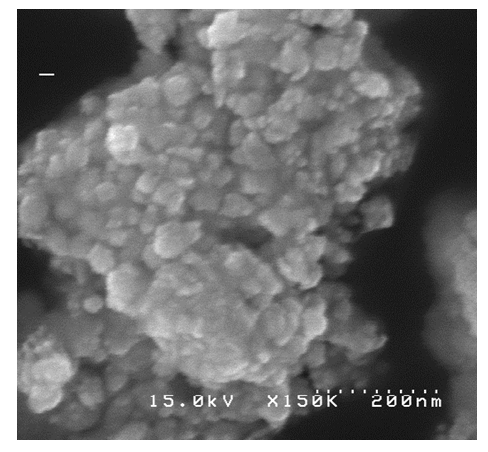 | Figure 9. SEM image of Pt-Pd/Nafion catalyst (a) before using HgSO4.H2O and (b) after using HgSO4.H2O |
4. Conclusions
- The decomposition efficiency of bimetallic Pt-Pd catalyst was studied under different experimental conditions. The influence of pH and other materials such as, halides, various metal oxides, various sulfate salts, and a surfactant (sodium dodecyl sulfate) was studied. Among the halide ions (F-, Cl-, Br- and I-), iodide ions increased the decomposition rate. At the same time, iodide ions are adsorbed on the Pd sites of the surface, which results deactivation of the catalytic sites. Other halide ions have little or no effect on H2O2 decomposition. Meanwhile, as sodium dodecyl sulfate poisoned the catalyst surface permanently, so it should also be avoided on hydrogen peroxide decomposition using a Pt-Pd surface. Effects of several metal oxides on H2O2 decomposition were also studied. Among the metal oxides (TiO2, ZnO, MnO2 and Fe2O3) only MnO2 increases the reaction rate but the others had no or little effect on H2O2 decomposition. Among the sulfate salts, NiSO4.7H2O merely increases the reaction rate whereas HgSO4.H2O poisoned the catalyst permanently.
ACKNOWLEDGEMENTS
- The authors acknowledge Prof. Masato Machida, Department of Nano Science and Technology, Kumamoto University, Japan for supplying required materials to prepare Pt-Pd films on Nafion membrane. Shahjalal University of Science and Technology is acknowledged for financial supports.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML