-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2019; 9(4): 109-114
doi:10.5923/j.chemistry.20190904.01

Coordination Complexes of Transition Metals and Schiff Base with Potent Medicinal Activity
A. C. Bhowmick1, Bikash Dev Nath2, M. I. Moim1
1Department of Chemistry, Mawlana Bhashani Science and Technology University, Santosh, Tangail, Bangladesh
2Department of Applied Chemistry, Graduate School of Natural Science and Technology, Okayama University, Tsushima, Okayama, Japan
Correspondence to: A. C. Bhowmick, Department of Chemistry, Mawlana Bhashani Science and Technology University, Santosh, Tangail, Bangladesh.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The ligand N,N'-bis(salicylidene)ethylenediamine has been synthesized from the condensation of salicylaldehyde with ethylenediamine and, after isolation in the solid forms, it has been complexed with Ni (II) and Zn (II) ions. During the complexation reaction with transition metal ions, the four dentate ligand is coordinated with the central metal atom by its two active donor atoms N and O and from 1H NMR, it is also established that C-H is activated with Ni (II) ions. The structures of the complexes have been elucidated by IR, 1H NMR and a combination of other analytical and physical measurements. UV-Visible spectrophotometer has been used to detect metals and specific absorption has been found for each metal. Some gram-negative bacteria such as E. Coli, Shigella Dysenteriae and Shigella Sonnei have been tested with metal complexes and a significant inhibition of bacterial growth by the metal complexes has been noted. To understand the antibacterial activity of these metal complexes streptomycin and ciprofloxacin have been used as standards during the experiment.
Keywords: Metals, Bacteria, NMR, Ciprofloxacin, Ligand
Cite this paper: A. C. Bhowmick, Bikash Dev Nath, M. I. Moim, Coordination Complexes of Transition Metals and Schiff Base with Potent Medicinal Activity, American Journal of Chemistry, Vol. 9 No. 4, 2019, pp. 109-114. doi: 10.5923/j.chemistry.20190904.01.
Article Outline
1. Introduction
- Nowadays, the use of transition metal complexes as medicine has been established as a powerful tool to fight against human diseases. That’s why, all over the world researchers are trying to develop suitable metal complexes that may be used as effective medicine in near future. For human, metal complexes may be used as an anticancer [1-3], antitumor [4-7], antiviral [8], and antimalarial [9] and antioxidant [10-16] agents. Alternately, metal complexes are also trying to apply for capturing images inside the infected organs of human body, instead of using expensive instruments. For example, novel bifunctional chelators (BFCs) containing 1,4,7-triazacyclononane or pyridinophane macrocycles and amyloid-binding 2-phenylbenzothiazole fragments have been synthesized, and their copper coordination properties have been characterized and applied in PET imaging agents for Alzheimer’s disease [17]. In addition to that, some coordination complexes have been explored for biological applications and have been found to interact with DNA, and function as drug delivery vectors [18]. This provides an overview of the 3D structures of coordination complexes that makes the complexes suitable for biomedical applications in future. Alternately, some suitable complexes have been used more precisely, as BODIPY derivatives, for the elaboration of BODIPY-based theranostics [19], multimodal imaging probes, and photodynamic therapy sensitizers. So, by the modification of the ligand with the active coordinating atoms, it is possible to tune the medicinal activity of coordination complexes. For example, recently some coordination complexes of thiosemicarbazones, semicarbazones, hydrazide/hydrazones and dithiocarbamates with strong pharmacological properties have been synthesized and published [20]. Recently, the thiosemicarbazones have achieved considerable attention by medicinal chemists since these are the excellent chelators of transition metals alongside their potential antitumor activity in vitro and in vivo [21,22].Along with their medicinal activities, metal coordination complexes have also been studied extensively for applying as antibacterial [23,24] and antifungal [25,26] agents since infectious diseases are spreading worldwide and the microorganisms are showing resistant against most of the traditional drugs [27-29]. For this reason, these compounds are being thought to be used in many commercial products such as soaps, detergents, household cleaners, paints, kitchenware, and school and hospital utensils [30] as inhibitors. Besides medicinal and antibacterial activities, over the last few decades the coordination chemistry has been emerged as a very nice tool for research due to their significant applications in various fields such as luminescent studies [31], solar cells technology [32,33], cytotoxicity studies [34], and as potent catalysts [35-38]. For example, surprisingly a bis-dithiocarbamate nickel complex has been recently tested for the photo catalytic production of hydrogen [39,40]. Moreover, as a potent pesticide, the applications of coordination compounds [41,42] are increasing day by day in plant biological system as rodenticidal, herbicidal, insecticidal, anthelmintical and plant-growth regulator to control unwanted organisms. Nonetheless, the transition metal complexes, derived from Schiff bases have occupied a central role in the development of coordination chemistry. Schiff base named after Hugo Schiff (1864) are the compounds containing azomethine group (-HC=N-) formed by the condensation reaction of any primary amine with aldehyde or ketone under specific condition [43]. Schiff bases are the nitrogen analogue of aldehyde or ketone in which the active carbonyl group (>C=O) is replaced by an imine or azomethine group. These bases are the effective chelating due to the presence of potentially coordinating functional groups near the site of condensation. Schiff bases derived from hydrophobic S-alkyl/aryl groups and their complexes reveal promising potential applications [44] in every field. For example, in biological cycle Zn (II) and Cu (II) Schiff base complexes [45-49] are being used for DNA binding and DNA cleavage. Finally, various viruses and bacteria are being reported to introduce a lot of problem to human body along with severe infections which are very difficult to cure and ultimately, the infected person dies due to the poisonous or toxic effect. That’s why, the researchers are trying to develop some Schiff base coordination complexes as the potent inhibitors of those microbial growths [50]. Keeping these facts in mind and in view of the significance of metal complexes in medicine (Figure 1), here we report the synthesis and characterization of some Schiff base coordination complexes of Ni (II) and Zn (II) ions.
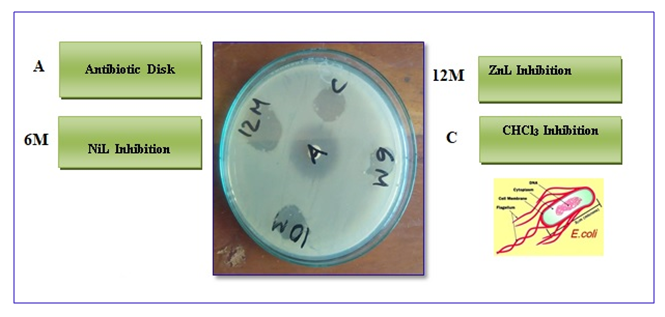 | Figure 1. Antibacterial activity (Inhibition of bacterial growth) |
2. Experimental
2.1. Materials and Instruments
- All the chemicals used were analytical grade. The molar conductance was measured by Elico-Conductometer. The IR spectra were recorded on Perkin-Elmer FTIR spectrophotometer in KBr pellets. The UV-visible spectra were recorded in CDCl3 and CH3OH solvent on Beckman DU-64 spectrophotometer with quartz cells of 1 cm path length. 1H NMR spectra were recorded in CDCl3 solvent in a Bruker Advance 400 MHz instrument. Elemental analysis was done in Vario EL Cube semi macro and micro elemental analyzer (Germany). Melting point was determined by Fisher melting point apparatus (Dimension 35×20×45, up to 350°C).
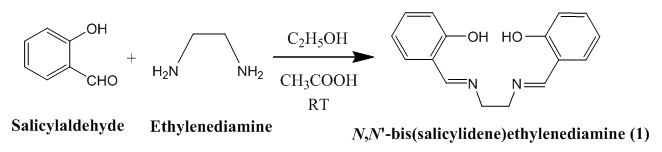
2.2. Synthesis of Schiff base [C16H16N2O2] (L)(1)
- 30 mL acidic (1 mL glacial acetic acid) ethanolic solution of salicylaldehyde (8.1412 g, 66.66 mmol) was stirred for 10 min and to this solution ethylenediamine (2 g, 33.28 mmol) was added drop wise and finally, yellow N, N'-bis(salicylidene)ethylenediamine was collected by filtering (Scheme 1). The resulting crude product was recrystallized in CHCl3 at room temperature. Spectral data: Anal. Cal. For C16H16N2O2: C, 71.56; H, 5.26; N, 10.44. Found: C, 71.69; H, 5.99; N, 10.50. IR (υ CO, KBr): 3455 (OH, b), 3052 (ArC-H, w), 3014 (N=C-H, w), 2937 (CH2, w), 2860 (CH2, w), 1636 (C=N, vs), 1578 (ArC=C,s), 1198 (C-C, s), 1283 (C-O, m), 1148 (C-N, vs). 1H NMR (CDCl3): δ 13.2 (2H, s), δ 7.3-6.9 (8H, m), 8.4 (2H, s), 3.9 (4H, s).
 | Scheme 1. Structure of Schiff base ligand, L= [C16H16N2O2] |
2.3. Synthesis of [NiL] (2)
- 0.4905 g (1.87 mmol) of NiSO4.6H2O was dissolved in 20 mL DMSO and stirred for 30 min with a magnetic spin bar and in another pot, 0.500 g (1.87 mmol) of N, N'-bis(salicylidene)ethylenediamine was also dissolved in 10 mL DMSO and it was added to the metal salt solution and the resulting solution was refluxed for 4 h and finally, reddish brown crystal was separated (Scheme 2) and recrystallized in CHCl3. Spectral data: Anal. Cal. For C16H14N2O3Ni: C, 56.31; H, 4.14; N, 8.21. Found: C, 57.58; H, 4.41; N, 8.35. IR (υ CO, KBr): 3436 (H2O-OH, b), 3052 (ArC-H, w), 2948 (CH2, w), 2852 (CH2, w), 1621 (C=N, vs), 1536 (ArC=C,s), 1198 (C-C, s), 1237 (C-O, m), 1145 (C-N, vs), 515 (Ni-N), 461 (Ni-O, m). 1H NMR (CDCl3): δ 7.0-7.4 (8H, m), δ 3.4 (4H, s), δ 1.6 (2H-H2O, s). UV- Visible (λmax, CHCl3): 253, 329, 412.
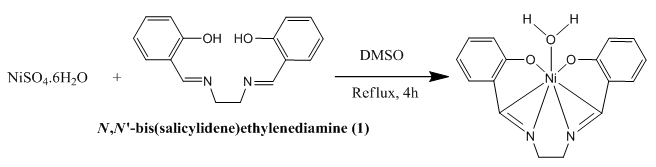 | Scheme 2. Structure of metal complex |
2.4. Synthesis of [ZnL](3)
- 20 mL solution of 0.5550 g (1.87 mmol) of Zn(NO3 )2. 6H2O in DMSO was stirred for 30 min and later, 0.500 g (1.87 mmol) of N, N'-bis(salicylidene)ethylenediamine solution in DMSO was added to the metal salt solution and the resulting solution was refluxed for 4 h and finally, yellowish crystal was separated (Scheme 3) and recrystallized in MeOH. Spectral data: Anal. Cal. For C16H14N2O2Zn: C, 57.89; H, 4.25; N, 8.44. Found: C, 60.86; H, 4.13; N, 6.73. IR (υ CO, KBr): 3424 (free H2O-OH, b), 3048 (ArC-H, w), 3018 (N=CH, w), 2922 (CH2, w), 2848 (CH2, w), 1632 (C=N, vs), 1532 (ArC=C, s), 1187 (C-C, s), 1237 (C-O, m), 1145 (C-N, vs), 531 (Zn-N, m), 465 (Zn-O, m). 1H NMR (CDCl3): δ 6.6-7.2 (8H, m), δ 8.5 (2H, s), δ 3.9 (4H, s). UV-Visible (λmax, MeOH): 225,261, 349.
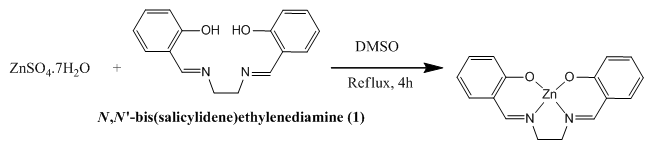 | Scheme 3. Structure of metal complexes |
2.5. Analysis of Anti-Bacterial Activity
2.5.1. Preparation of Nutrient Agar media
- 7.9 g of Nutrient agar was dissolved in 250 mL distilled water and after shaking, it was put in autoclave for ½ h at 121°C. The resulting solution was poured in eight plates (disks) and rested the plates for 10 min. 10 μl E. coli gram negative bacteria was applied to all disks uniformly and the standard antibiotic streptomycin and ciprofloxacin were put at the center of the plate. In a test tube, 1 mg metal complexes were dissolved by 1 mL of CHCl3 and 10 μl of the sample was dropped into the prepared bacterial disk (Figure 1). The solvent CHCl3 may also inhibit the bacterial growth and, that’s why, in a separate disk 10 μl CHCl3 was dropped to observe the probable inhibition. Same procedure was repeated for Shigella Dysenteriae and Shigella Sonnei gram negative bacteria.
3. Result and Discussions
3.1. Physical Properties
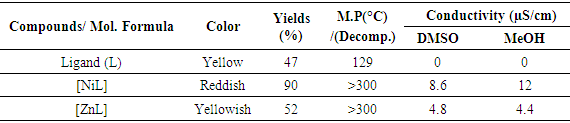
- All the synthesized ligand and metal complexes were air stable (Table 1). Complexes are colorful and crystalline. Molecular conductance values are (3-12 µS/cm) indicating that complexes are poor electrolyte and melting point is above 300°C, except the Schiff base ligand 129°C. From the conductivity measurement it is possible to predict that nonionic complexes were formed, but the conductivity of all complexes is zero in CHCl3. Every metal complex gave very good (λmax) UV-Visible absorption.
|
3.2. Infrared Spectra
- The chemical reactions between the Schiff base ligand (L) and metals are shown in the Scheme 2 and Scheme 3. Spectroscopic data are shown in experimental section (2.2-2.4). In IR, the hydrogen stretching (=CH-) at 3014 cm-1 (L) and the C=N stretching at 1636 cm-1 (L) confirmed the formation of imine bond (-CH=N-) (Scheme 1) and no peak was found for aldehyde carbonyl group stretching. Some other IR stretching peaks at 3455 cm-1 and 3052 cm-1 came up for the phenolic O-H and aromatic hydrogens, respectively. For the aliphatic two CH2 group the C-H stretching frequencies found at 2937 cm-1 (symmetric) and 2850 cm-1 (asymmetric). In IR all metal complexes gave distinctive stretching band for each group. The stretching of water (-OH) found between 3444-3417 cm-1, aromatic hydrogen (Ar-H) 3075-3044 cm-1, imine hydrogen (N=CH-) 3020-3002cm-1, aliphatic hydrogens (-CH2-) 2983-2913 cm-1 (symmetric) and 2863-2827 cm-1 (asymmetric). Since phenolic -OH coordinated with metal, that’s why no band was detected for this group in IR and simultaneously, in Ni (II) complex no band was found for =CH-, since two =CH- activated with metal.
3.3. Proton magnetic Resonance Spectra
- In 1H NMR, the ligand N, N'-bis(salicylidene)ethlenediamine (1) gave distinctive chemical shift for proton. All Ar-H gave the δ between 6.9-7.3 ppm and it was also found δ for CH2 at 3.9 ppm, δ for imine hydrogens at 8.4 ppm, δ for phenol hydrogens at 13.2 ppm. The two CH2 group gave singlet due to their similar chemical environment. The two donor atoms N and O of the ligand (1) coordinated with metals Ni (II) (Scheme 2) and Zn (II) (Scheme 3) and exceptionally, the metal complex of the Ni (II), formed C-Ni bond [51,52], since in 1H-NMR no chemical shift was found for =CH-. Though, it appears exceptional, but it happens at high temperature, since the reaction was refluxed close to 4 h in DMSO (dimethyl sulfoxide) at 189°C. Metal complexes after dissolving in CHCl3, unreacted metal salt was separated by filtering off, since both ligand and metal complexes dissolve in CHCl3, that’s why excess metal salts were used so that unreacted ligand could not present in the reaction mixture. Finally, metal complexes were recrystallized in three times for getting pure crystal. The process was repeated for all complexes. In 1H NMR the metal complexes of Ni (II) and Zn (II) gave chemical shift values for aromatic protons between δ 6.9-7.6 ppm (multiplets) and imine hydrogens between δ 8.1-9.9 ppm. None of the complexes gave phenolic hydroxyl peak since phenolic hydroxyls formed complex with metals. Aliphatic protons gave δ between 2.4-2.6 ppm and coordinating water protons δ at 1.6 ppm. The number of protons was calculated according to proton integration. The Zn (II) complex did not give any chemical shift for coordinating water and that’s why the complex formed square planer complex with no coordinating water.
3.4. Antibacterial Activity
- The Schiff base ligand (N, N'-bis(salicylidene)ethlenediamine) and its metal complexes were screened for their antibacterial activity against the strains the E. Coli and Shigella Dysenteriae. The susceptibility zones were measured in diameter (mm) and the result are listed in (Table 2). The susceptibility zones were the clear zones around the discs killing the bacteria (Figure 1). The Schiff base and metal complexes individually exhibited varying degrees of inhibitory effects on the growth of tested bacterial species. Most of the metal complexes showed more antibacterial activity than Schiff base ligand. But, none of the complexes showed antibacterial activity against the growth of gram-negative bacteria Shigella Sonnei. In this experiment the antibiotic streptomycin was used as standard and the antibiotic also showed good activity against the growth of bacteria.
|
4. Conclusions
- The present study describes the synthesis and characterization of Ni (II) and Zn (II) complexes of N, N'-bis(salicylidene)ethlenediamine. The structures of the complexes were determined successfully by IR, 1H NMR spectroscopic techniques and elemental analysis. Based on the physico-chemical and spectroscopic data, we propose monodentate coordination of N, N'-bis(salicylidene)ethlenediamine to the metal, which is further confirmed by the UV-Visible absorption. In Ni (II) complex, the ligand coordinated to the metal atom through the imine carbon and an octahedral structure for Ni (II) was formed. Furthermore, the results of the antibacterial studies revealed drastic antibacterial effects of these complexes on the growth of gram-negative bacteria. However, further study is going on to get more potent antibacterial agents by synthesizing new metal coordination complexes.
ACKNOWLEDGEMENTS
- I am thankful to the Department of Chemistry in Mawlana Bhashani Science and Technology University (MBSTU) for supporting the research. I am also thankful to Abu Shibly, Assistant Professor, Department of Biotechnology and Genetic Engineering, MBSTU for helping the antibacterial analysis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML