-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2019; 9(3): 95-102
doi:10.5923/j.chemistry.20190903.02

Bioaccumulation of Heavy Metals in Parts of the Silver Catfish (Chrysichthys nigrodigitatus) Harvested from Badagry Creek and Fish Ponds in Badagry, Lagos, Nigeria
Osundiya Medinat Olubunm1, Olowu Rasaq Adewale1, Alegbe Monday John1, Igbasan Sam Odunayo2, Abayomi Abdulazeez Jimoh3, Olawoye Christiana Olawunmi1, Emmanuel Jesuyon1
1Department of Chemistry, Lagos State University, Ojo, Nigeria
2Centre for Environmental and Science Education, Lagos State University, Ojo, Nigeria
3Department of Fisheries, Lagos State University, Ojo, Nigeria
Correspondence to: Olowu Rasaq Adewale, Department of Chemistry, Lagos State University, Ojo, Nigeria.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In aquatic environment, heavy metals are produced from natural and anthropogenic sources and the degree of contamination in fish is dependent on the sampling site, pollution types as well as mode of feeding. The concentration of heavy metals such as nickel (Ni), zinc (Zn), iron (Fe) and cadmium (Cd) were analyzed in various parts (head, gills intestine, gill and tail) of the silver catfish, Chrysichthys nigrodigitatus which were collected from Badagry creek and different ponds namely Morogbo (MOP), Magbon (MAP), Churchgate (CGP) and Ajibade (AJP) ponds within Badagry axis. The concentration of the heavy metals was determined using atomic absorption spectrophotometer (AAS). The results showed the accumulation of the metal differently in various part of the fish. Heavy metals concentration in the fish sample was in this order: Zn > Fe > Ni > Cd. Zinc was abundant and had the highest level of mean concentration accumulated in the head and all parts of the fish samples from both the lagoon and the fish ponds with the highest value of 3.06 µg/g recorded in the intestine of the fish sample from Church Gate concrete pond. The high zinc value could be associated with the fact that this metal is naturally abundant in Nigerian soils and fish accumulate zinc from both the surrounding water, sediments and their diet but the concentrations are below recommended value of Food and Agriculture Organization (FAO) and Lagos State Environmental Protection Agency (LASEPA) for daily intake. Iron was the second most concentrated metal with a mean concentration ranging from 0.33 - 2.37 µg/g, and this may be attributed to the clay nature of Nigerian soils. Nickel concentration in the samples ranged from 0.14-2.98 µg/g which may due to the discharge of industrial effluents, domestic and agricultural activities around Badagry axis. Cadmium was not detected in samples from Morogbo fish ponds, but was detected in samples from the lagoon and the other three fish ponds, with values ranging from ND – 0.25 µg/g, and this was relatively below the recommended value of FAO and LASEPA in fish which is an indication that the C. nigrodigitatus from these sampling sites was safe for human consumption.
Keywords: Bioaccumulation, Ponds, Lagoon, Fish part, Deposition, Anthropogenic and aquatic ecosystem
Cite this paper: Osundiya Medinat Olubunm, Olowu Rasaq Adewale, Alegbe Monday John, Igbasan Sam Odunayo, Abayomi Abdulazeez Jimoh, Olawoye Christiana Olawunmi, Emmanuel Jesuyon, Bioaccumulation of Heavy Metals in Parts of the Silver Catfish (Chrysichthys nigrodigitatus) Harvested from Badagry Creek and Fish Ponds in Badagry, Lagos, Nigeria, American Journal of Chemistry, Vol. 9 No. 3, 2019, pp. 95-102. doi: 10.5923/j.chemistry.20190903.02.
Article Outline
1. Introduction
- Heavy metals belong to the group of elements whose hydro-geochemistry cycles have been deeply hastened by man. The fast industrialization, coupled with technological advances in agriculture, has launched various pollutants both synthetic and organic into the aquatic ecosystems, which serves as the ultimate sink for most metals. Consequently the levels of heavy metals in fish usually reflect levels found in sediment and water of the particular aquatic environment from which they are sourced [1-3]. The consequence of increased metal loadings in aquatic ecosystems is coincidental with acidification and fish population losses were a significance of reproduction failures arising from both acid and metal stresses [4]. Fish is widely consumed by both the low and high income earners because of the easy accessibility and the silver catfish (Chrysichthys nigrodigitatus) is an important source of protein and are bio-indicators of metal pollution [4-6]. The pollution of the aquatic environment with heavy metals has become a global problem during recent years, because they are indestructible and most of them have toxic effects on organisms [2,5,6]. Among environmental pollutants, metals are of particular concern due to their potential toxic effect and ability to bio-accumulate in aquatic ecosystems [2-6]. There is increasing concern about the quality of foods globally and the determination of toxic elements in food has prompted studies on toxicological effects of these metals in food [7]. Heavy metals are considered the most important form of pollution of the aquatic environment because of their toxicity and accumulation by marine organisms [4,5-8] and they act as one of the most serious pollutants in our natural environment due to their toxicity, persistence and bioaccumulation problems [8,9]. Most heavy metals have no beneficial functions to the body and can be highly toxic. If they enter into the body through inhalation, ingestion and skin they accumulate in the body tissue faster than the body’s detoxification pathways can dispose of them [2,5,9,10]. High concentration exposure is not necessary to produce a state of toxicity in the body tissue and, overtime, can reach toxic concentration at low levels [11]. Even though each metals exhibit specific signs of their toxicity, the following have been reported as general signs associated with cadmium, lead, arsenic, mercury, zinc, copper and aluminium poisoning: gastrointestinal (GI) disorders, diarrhea stomatitis, tremor, hemoglobinuria causing a rust–red color to stool, ataxia, paralysis, vomiting and convulsion, depression, and pneumonia when volatile vapors as well as fumes are inhaled and the nature of effects could be toxic (acute, chronic or sub-chronic), neurotoxic, carcinogenic, mutagenic or sub-chronic), neurotoxic, carcinogenic, mutagenic or teratogenic [12]. Long-term exposure may result in slowly progressing physical, muscular, and neurological degenerative processes that mimic Alzheimer’s disease, Parkinson’s disease, muscular dystrophy, and multiple sclerosis [2,5]. Fish has been reported to accumulate large amounts of some metals from the water and are often at the top of the aquatic food chain [12,13]. Consumption of fish are generally observed in both the low and high income earners because of the easy accessibility through free fishing methods in open lakes and rivers as well as low saturated fat, protein content and the presence of omega fatty acids known to support good health [5,13]. The inflow of contaminated water sources into water body or an aquatic ecosystem has a deleterious consequence on microorganisms, plants and animals that depend on water for life, more so aquatic organisms of fishes are paramount [5,12] Potential toxic metal (PTM) that find their way into surface water exists in colloidal, particulate and dissolved phase, although dissolved concentrations are generally low [13,14]. The colloidal and particulate metal may be found in hydroxides, oxides, silicates or sulphides and the soluble forms are generally ions or unionized organometallic chelates or complexes [14]. The solubility of trace metals in surface waters is predominantly controlled by the water pH, the type of and concentration of ligands on which the metal could absorb, and the oxidation state of the mineral components and the redox environment of the system [14]. However, Tchounwou et al [15] reported that metals such as cobalt (Co), copper (Cu), chromium (Cr), iron (Fe), magnesium (Mg), manganese (Mn), molybdenum (Mo), nickel (Ni), selenium (Se) and zinc (Zn) are essential nutrients that are required for various biochemical and physiological functions and that inadequate supply of these micro-nutrients results in a variety of deficiency diseases or syndromes. Their bioavailability is influenced by physical factors such as temperature, phase association, adsorption and sequestration [15]. With fast industrialization and entrepreneurial life style, sources of environmental pollution have increased. The pollution occurs both at the level of industrial production as well as end use of the products and run-off. These toxic elements enter the human body mostly through food and water and to a lesser extent through inhalation of polluted air, use of cosmetics, drugs, poor quality herbal formulations and even items like toys which have paints containing lead [2,21]. This work is geared toward assessing the status of these heavy metals in C. nigrodigitatus from Badagry creek and surrounding ponds in Badagry area of Lagos State from which fishes consumed are harvested.
2. Materials and Methods
2.1. Study and Sampling Area
- The sample sites are located in Badagry area in Lagos State, Nigeria. The Badagry creek and the ponds around its axis is approximately 60km long and 3km wide, and lies between longitudes 3°0′ and 3°45′E and latitudes 6°25′ and 6°30′N. Its water depth ranges from 1m to 3m. Most periods of the year, the Badagry creek is characterized by fresh and slightly brackish water while some of the ponds are constructed on a swampy area (earthen pond) with some using concrete. Fish samples were collected from Ajibade down to Badagry due to the industries around its axis, domestic waste from the environment and the type of feeds used in feeding the fishes. The fish sample used for analysis was the silver catfish (Chrysichthys nigrodigitatus), from the sampling areas. The locations of ponds used for this study were Ajibade, Churchgate, Magbon and Morogbo, and Badagry creek within Badagry axis of Lagos State (Figure 1).
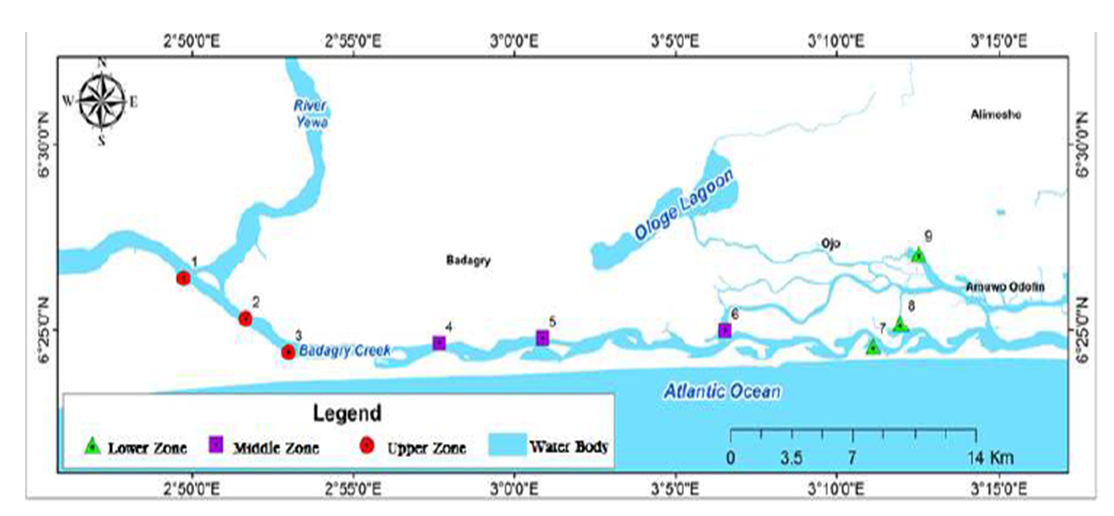 | Figure 1. Badagry Creek and other Sampling Sites |
2.2. Sampling Methods and Preparation
- (a) Catfish Sampling and PreparationFish samples from the lagoon were collected between the period of September to November 2017 from Badagry creek and ponds (earthen and concrete) around its axis. Sampling was carried out in accordance with the recommendations of UNEP reference method for marine pollution studies [21]. The species used for the study was the silver catfish (Chrysichthys nigrodigitatus). Samples of the fish were caught at Badagry lagoon with the assistance of fishermen around its axis using drag net, which were usually left over night in the lagoon by local fishermen. The netted fish were recovered each morning in a transparent plastic and that of the ponds were collected by the fish farmers using a drag net. Thereafter, the fish samples were transported on ice to the laboratory.Identification of the fish was done in the Department of Fisheries, Lagos State University. Each fish was properly cleaned by rinsing with distilled water to remove debris, planktons and other external adherents. It was then drained under folds of filter, weighed, wrapped in aluminum foil and then frozen at 10°C prior to analysis [13].For analysis, fish samples were defrosted in batches for two hours and then weighed. Each sample was then cut and separated into head, trunk, gills, intestine and tail using a plastic knife [13,21]. The different fish parts from the lagoon and ponds were dried at 80°C for 2h in Gallenkamp hot box oven and then blended in an electric moulinex blender. The fish samples were prepared for metal analysis using the dry digestion method.Approximately 2.0 g each of sample was weighed and ashed in the furnace at 550°C for 90 minutes with the aid of a crucible. The ash was dissolved in 5 mL of concentrated nitric acid and made up to 25 mL volume with distilled water. The heavy metals were analyzed using BUK Scientific Model 210 Atomic Absorption Spectrophotometer.Statistical AnalysisData generated were analyzed statistically by calculating the mean and standard deviation of the measured parameters.
3. Results and Discussion
3.1. Results
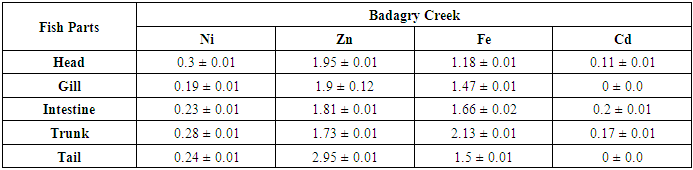
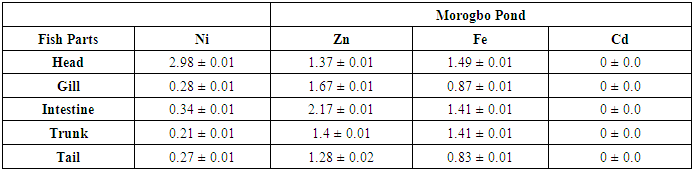
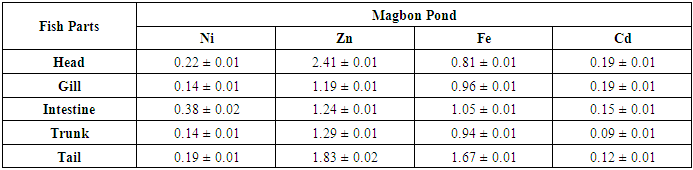
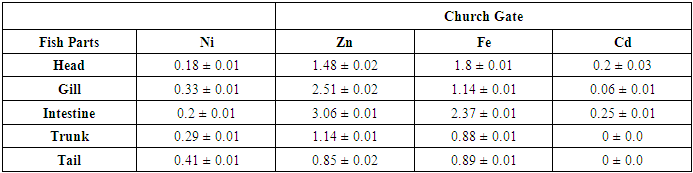
- The mean concentrations ((µg/g)) and standard deviation of potential toxic metals nickel (Ni), zinc (Zn), iron (Fe) and cadmium (Cd) in the catfish samples cllected from Badagry creek and fish ponds located at Morogbo (MOP), Magbon (MAP), Churchgate (CGP) and Ajibade (AJP) ponds which are earthen and concrete ponds are presented in Tables 1-5.
|
|
|
|
|
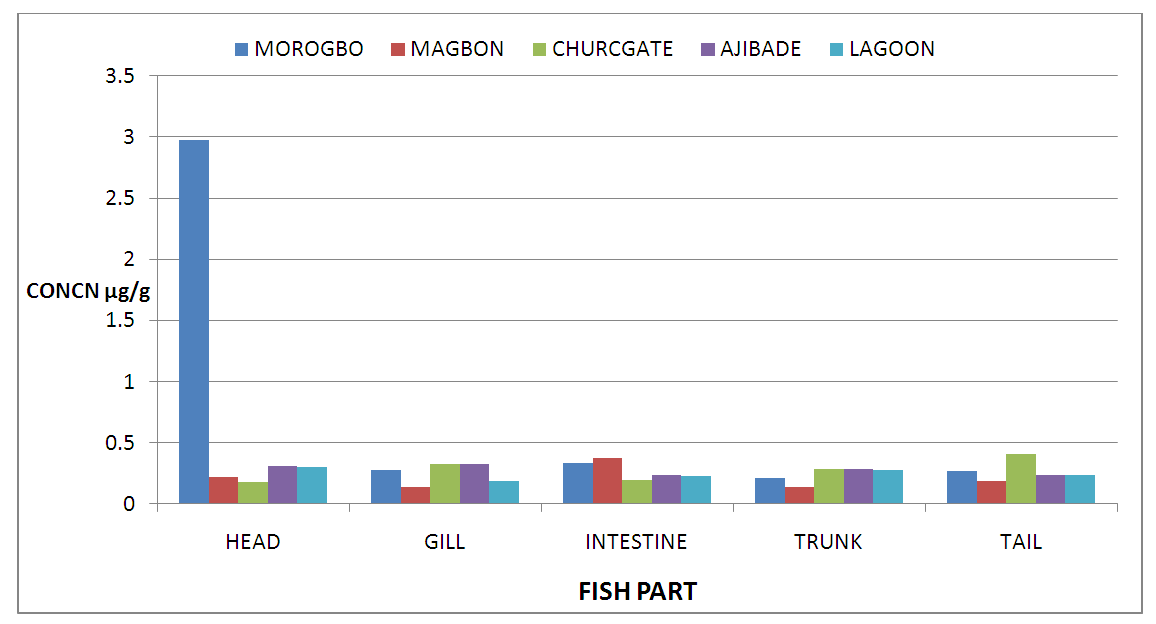 | Figure 2. Concentration of Nickel (Ni) in Fish Parts from Badagry Creek and Surrounding Ponds |
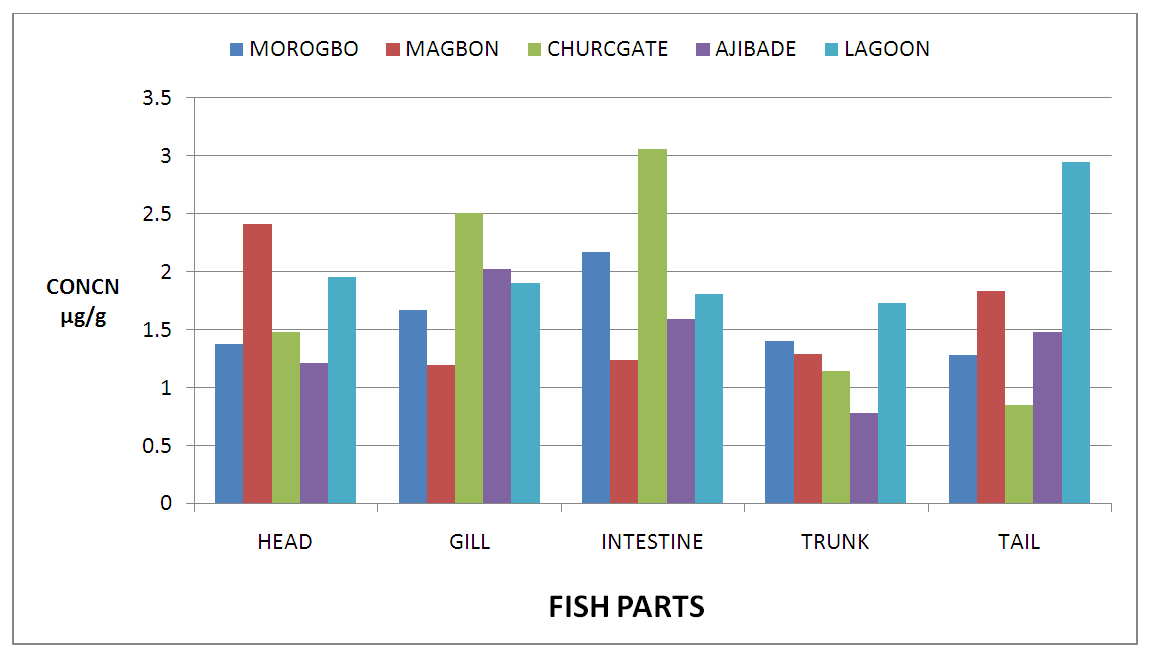 | Figure 3. Concentration of Zinc (Zn) in Fish Parts from Badagry Creek and Surrounding Ponds |
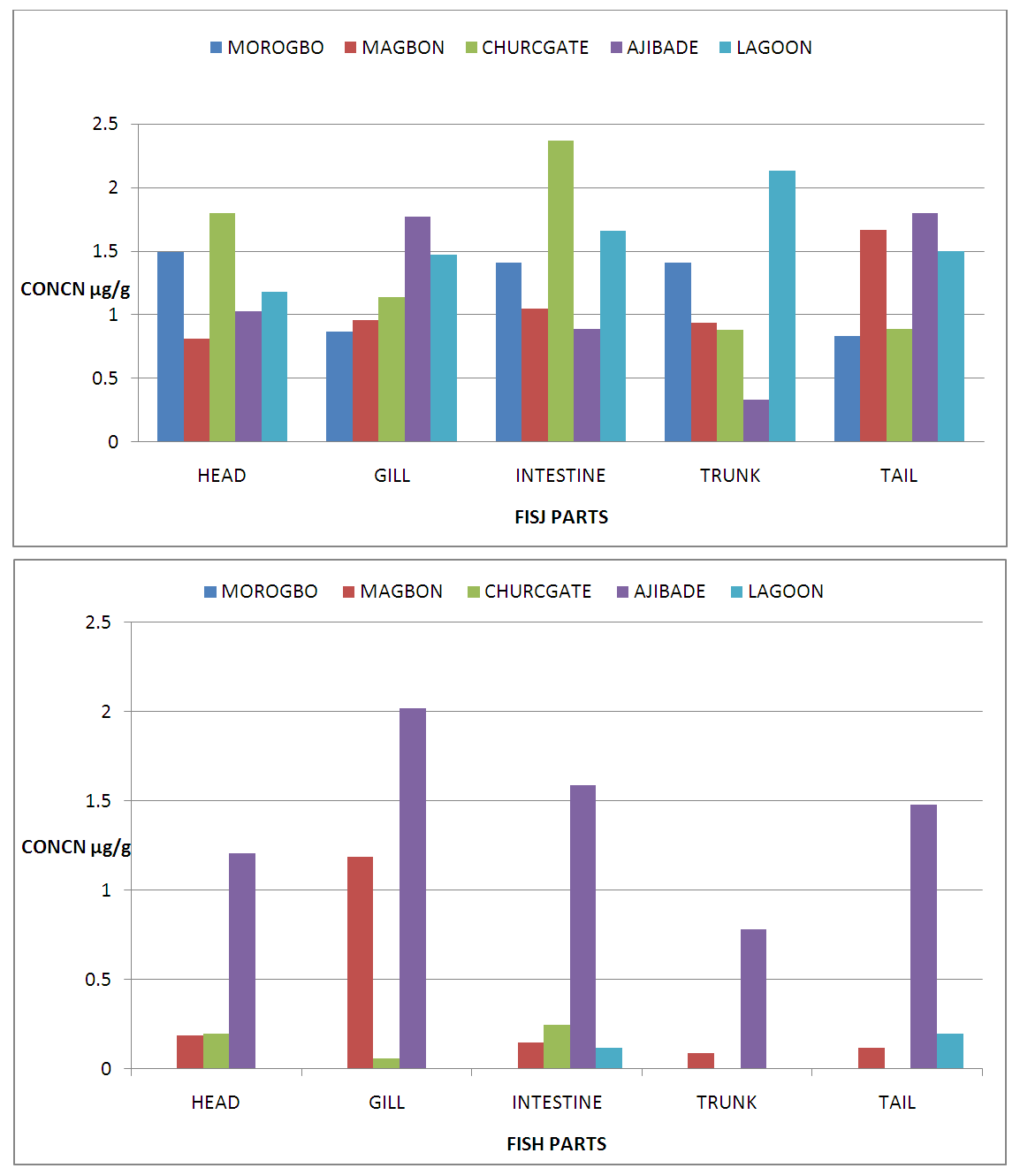 | Figure 4. Concentration of Iron (Fe) and Cadmium (Cd) in Fish Parts from Badagry Creek and Surrounding Ponds |
3.2. Discussion
- Pollution of aquatic environment with chemical pollutants especially heavy metals that are persistent in nature have become one of the most critical problems of the century. Fish can be considered as a good bio-indicator for heavy metal contamination in marine ecosystem because they are present in different trophic level and are widely consumed globally by human [6,13,20]. In the river, fish are often at the top of the food chain and have the affinity to concentrate heavy metals from water. Consequently, bioaccumulation of metals in fish can be considered as an index of metal pollution in the aquatic bodies [8]. Heavy metal whether essential or not may be toxic to living organism including fish and this can lead to various deleterious effect on fisheries and potentially public health [6,2,13]. In this study the concentration of some selected heavy metal Nickel (Ni), Zinc (Zn), Iron(Fe) and cadmium(Cd) in the head, gills, intestine, trunk and tail in the silver catfish (Chrysichthys nigrodigitatus) harvested from Badagry creek and ponds in Badagry, Lagos south west Nigeria were examined. The results of this study showed that Ni, Zn, Fe and Cd were all present in different concentrations in the various part of the fish except cadmium which was not detected in the Morogbo pond which agreed with earlier report [2,5,13,21]. Nickel was detected in the Badagry creek, Morogbo, Magbon, Churchgate and Ajibade ponds and the level of nickel (Ni) in the fish sample was high especially in the Morogbo pond fish head which was followed by tail from Churchgate and intestine from both Badagry creek and Magbon ponds sample which correlated with the finding of Olowu et al [21]. The high concentration of nickel may be attributed to anthropogenic activities, high vehicular activities as well as increase in the discharge of liquid and solid fuel into the water body from domestic waste [22], but the values in the fish sample were less than what was reported by Turkmen et al. [23]. Ni may be ingested directly through the gills or indirectly from food and may be taken up from the sediment, a major depository of heavy metal. The level of nickel in the head, gill, intestine and trunk and tail from the sampled are below the daily recommended standard [24-27]. The order of nickel accumulation in the fish sample was head> tail> intestine > gills >trunk. The level of concentration of nickel in head and gills may be attributed to the fact that water passes through the mouth and gills as reported by Olowu et al. [2,13,21]. In this study, Zinc was present in all the fish harvested from both the lagoon and ponds within Badagry axis but the highest concentration value of 3.06 µg/g was recorded in the intestine from Church gate concrete pond compared to others with the lowest concentration of 0.06 µg/g recorded in the fish gills of the same pond which is in agreement with earlier finding [2,5,8,13], but differs from what was obtained by Bat et al. and Turkmen et al respectively [6,23].Zinc was the most concentrated metal in all the catfish parts sampled, with the highest mean concentration recorded in the head and gills which correlates with of other findings [2,13]. Fish can accumulate zinc from both the surrounding water and from their diet. Although zinc is an essential element, at high concentrations, it can be toxic to fish, cause mortality, growth retardation, and reproductive impairment. Zinc is capable of interacting with other elements and producing antagonistic, additive or synergistic effects [8]. The higher concentration of zinc recorded in the fish sampled from both the creek and the ponds could be attributed to human activities and vehicular movement that takes place around the sampling area. Human activities such as the use of chemicals, zinc-based fertilizer, and petrochemicals such as discarded engine oil, welder and automobile mechanic workshops could also enhance a high concentration of this metal in the soil and surrounding water [13]. The concentration level of heavy metal recorded in the fish part was generally low when compared to WHO/FEPA/EU recommended standards [24,27,29-31].Iron was detected in all the samples harvested from both creek and ponds with highest concentration observed in the intestine of the fish part which was closely followed by fish trunk from the Badagry creek samples. The high mean value of Fe obtained in the analyzed sample may be attributed to the clay nature of Nigerian soil which is in agreement with the findings of earlier researchers [13,19,21]. Iron (Fe) as an essential element is involved in the synthesis of hemoglobin in the red blood corpuscles of blood as well as assists in the production of red blood cells [13]. In this study, the observed mean value of Fe in the fish parts were less the recommended limit as well as less than what was reported by Olowu and it co- workers [21,24,29,30]. Cadmium was detected in all the samples analyzed except in the fish part sample from Morogbo ponds, but the concentrations were below the recommended value 0.5 µg/g [24,25] which differs from what was reported by Turkmen et al [23] and cadmium like all other potential toxic metals could be absorbed through the gills and has been well known to cause hazard to fish gills. High exposure to cadmium can result to renal damage, anemic, cancer of the lung and bone disorder [13,23]. Cadmium concentrations reported from this study were below the WHO/FEPA maximum recommended limit of 0.5 µg/g in fish food and the levels were also low in comparison to 0.270 µg/g and 0.28 µg/g for fishes of the river Niger and black sea fish Mugilauratus from Sinop-Iccliman Turkey [27,28] which implies that the silver catfish (Chrysichthys nigrodigitatus) from the Badagry creek and the surrounding ponds investigated are safe for human consumption.
4. Conclusions
- The results of this study present a valuable baseline data on the heavy metals in the catfish (Chrysichthys nigrodigitatus) from Badagry creek and surrounding ponds investigated. The study revealed that the concentrations of heavy metals recorded in the catfish samples were below the WHO and FEPA recommended limits which are an indication that the fish from the studied areas are safe for human consumption.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML