-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2019; 9(3): 91-94
doi:10.5923/j.chemistry.20190903.01

The Chemistry of the Folin Test for Uric Acid
Francisco Sánchez-Viesca, Reina Gómez
Organic Chemistry Department, Faculty of Chemistry, National Autonomous University of Mexico, Mexico City (CDMX), Mexico
Correspondence to: Francisco Sánchez-Viesca, Organic Chemistry Department, Faculty of Chemistry, National Autonomous University of Mexico, Mexico City (CDMX), Mexico.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In the interaction of uric acid with sodium tungstate in phosphoric acid, followed by alkalinisation in order to develop a blue colour (Folin Test for uric acid) there are two reaction series, organic and inorganic. These are described and a mechanism has been provided for each reaction along with sustaining references to similar chemical deportment. The process is an oxidative degradation that involves addition to double bond, organometallic ester, oxido-reduction step, epoxide formation, pyrimidine ring breakdown, hydration, decarboxylation, and oxirane isomerization. The final product, 2,4-dioxo-5-ureido-imidazolidine, has been obtained before by other methods differing in oxidant and reaction medium, with no mechanism advanced. The chemistry related to the involved inorganic compounds is also treated in detail.
Keywords: Allantoin, Allophanic acid, Isocyanate, Oxidative degradation, Phosphotungstic reagent, Reaction mechanism, Reactive intermediates, Uric acid
Cite this paper: Francisco Sánchez-Viesca, Reina Gómez, The Chemistry of the Folin Test for Uric Acid, American Journal of Chemistry, Vol. 9 No. 3, 2019, pp. 91-94. doi: 10.5923/j.chemistry.20190903.01.
1. Introduction
- Continuing our studies on organic reaction mechanisms [1-4], we turned our attention to the Folin Test for uric acid.Besides the laboratory results, many times the colour tests induce the theorist’s interest in order to disentangle the reactions that are occurring.The clinical significance of uric acid is well known. At high uric levels (hyperuricemia) uric acid crystallizes and the crystals deposit in joints, tendons and surrounding tissues resulting in an attack of gout.In this paper we study the reaction mechanism of the Folin test, the reaction of uric acid with sodium tungstate in phosphoric acid that yields an intense blue colour [5]. We describe the mechanism of the steps of the uric acid oxidative degradation to allantoin and the coloured tungsten derivatives that are formed.There is a memorial article dedicated to Otto Folin [6]; and the reagent, though not complicated, is also commercially available (Supplier: VWR Chemicals).The discussion has been enhanced with logical connectors, similar reactivities, and 3D molecular structures.
2. Antecedents
- As said before, Folin’s reagent employs sodium tungstate in phosphoric acid. Closely related inorganic reagents that also give blue coloured products are the Buckingham reagent for alkaloids [7, 8] and the Froehde reagent [9, 10]. The first uses ammonium molybdate in concentrated sulphuric acid. The second utilizes sodium molybdate in the same acid, and Froehde employed it in a test for morphine. Years later the Irish physician E. W. Davy published a new test for carbolic acid (phenol) employing the sulphomolybdic reagent [11-13]. This discloses that the phenol group in morphine is responsible for the positive reaction of this compound.Molybdene and tungsten are transition elements that are vicinal in the d-block, group VI (the chromium group). Molybdates and tungstates are mild oxidants.Tungsten (Z=74) is in the third series and its electron structure is: Xe core, 4f14, 5d4, 6s2 [14]. Electron transfer from the 6s to the 5d orbitals gives d6 orbitals with additional stability. The hexavalent tungstates are example of this.Acidified tungstates when reduced give blue pigments and colorations [15]. When the acidified tungstate solution is reduced with hydrazine, hydrogen sulphide, or other reagents, an amorphous intense blue product is obtained [16].The blue products are W2O5 [17], and W3O8 [18], and both come from WO2, tungsten(IV) oxide (tungsten suboxide).This pertinent information will sustain the reaction mechanism proposed in the next section.
3. Discussion
- Uric acid is tautomeric and presents the lactam form (2,6,8-trioxopurine) and the lactim form (2,6,8-trihydroxypurine), Figure 1.
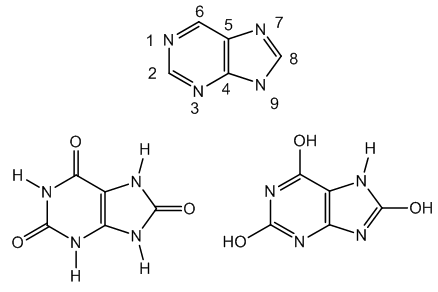 | Figure 1. Purine frame, and uric acid lactam and lactim forms |
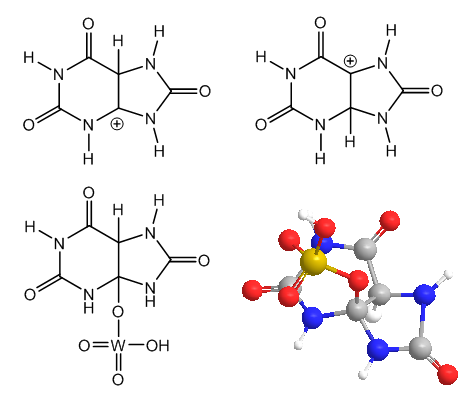 | Figure 2. Uric acid preferred and discarded protonations, and tungstic ester (2D- and 3D structures) |
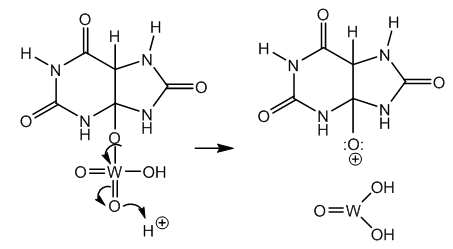 | Figure 3. Ester protolysis, oxonium ion intermediate and tungsten oxide hydrate |
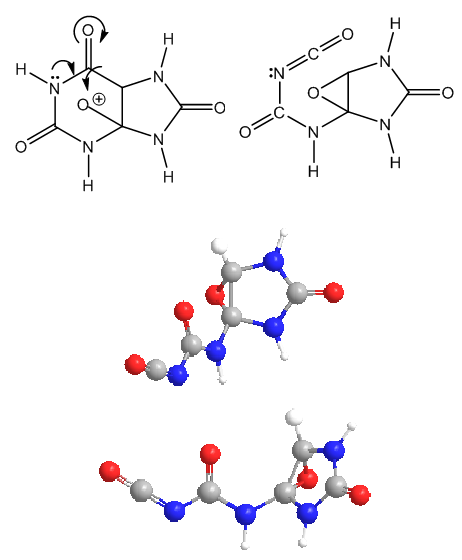 | Figure 4. Epoxide formation and pyrimidine ring opening, with two 3D-structures of the resulting molecule |
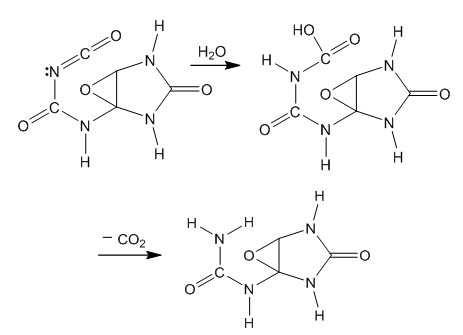 | Figure 5. Hydration and decarboxylation of the intermediate isocyanate |
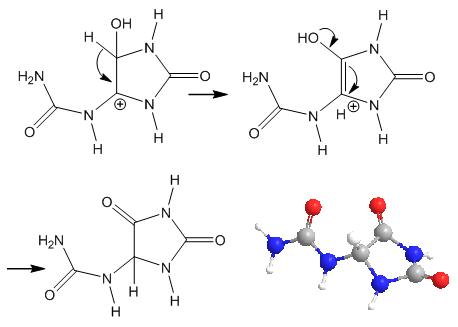 | Figure 6. Steps after epoxide opening and end product (2D and 3D) |
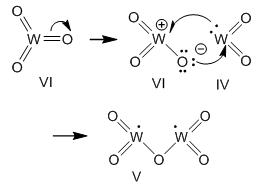 | Figure 7. Formation of tungsten pentoxide from tungstic anhydride and tungsten dioxide |
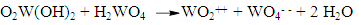 Then transfer of one electron from a neutral molecule of tungsten dioxide (in Fig. 7) to the above oxycation gives two radicals WO2+.Thus W3O8 is a salt, a tungstate anion and two oxycations with tungsten (V).
Then transfer of one electron from a neutral molecule of tungsten dioxide (in Fig. 7) to the above oxycation gives two radicals WO2+.Thus W3O8 is a salt, a tungstate anion and two oxycations with tungsten (V).4. Conclusions
- Although many colour tests are known since many years the reactions involved in them are frequently unknown. Sometimes there are hints, but no more. It is the theorist who unravels what is happening, step by step, in the reaction medium. This is the case with the Folin test for uric acid. What has been done is provide a complete reaction mechanism that involves both the organic chemistry of the tested substance and the inorganic chemistry related to the employed reagent, as well as the participation of the reaction medium.The reaction series includes the following intermediates: an organometallic ester, an epoxide, an isocyanate, an allophanic acid, an alcohol and an imide.The occurring reactions are: addition to double bond, mixed ester formation, protolysis, oxidative degradation involving epoxide formation and breakdown of the purinic pyrimidine ring, hydration, decarboxylation, a second protolysis, and isomerization.As it can be seen, it is rather a complex process. However, the final product is in agreement with experimental results obtained with other oxidizing agents and different reaction mediums.The provided mechanism was inferred from well known reactivities, i.e., from the chemical deportment of the involved functional groups (nomothetic explanation, by means of general laws or principles).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML