-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2019; 9(2): 71-90
doi:10.5923/j.chemistry.20190902.03

Synthesis and Catalytic Activity of Palladium Mediated Metallodendrimer for the Sonogashira and Heck Coupling Reactions
Md. Sayedul Islam, Md. Wahab Khan
Department of Chemistry, Faculty of Engineering, Bangladesh University of Engineering and Technology (BUET), Dhaka, Bangladesh
Correspondence to: Md. Sayedul Islam, Department of Chemistry, Faculty of Engineering, Bangladesh University of Engineering and Technology (BUET), Dhaka, Bangladesh.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

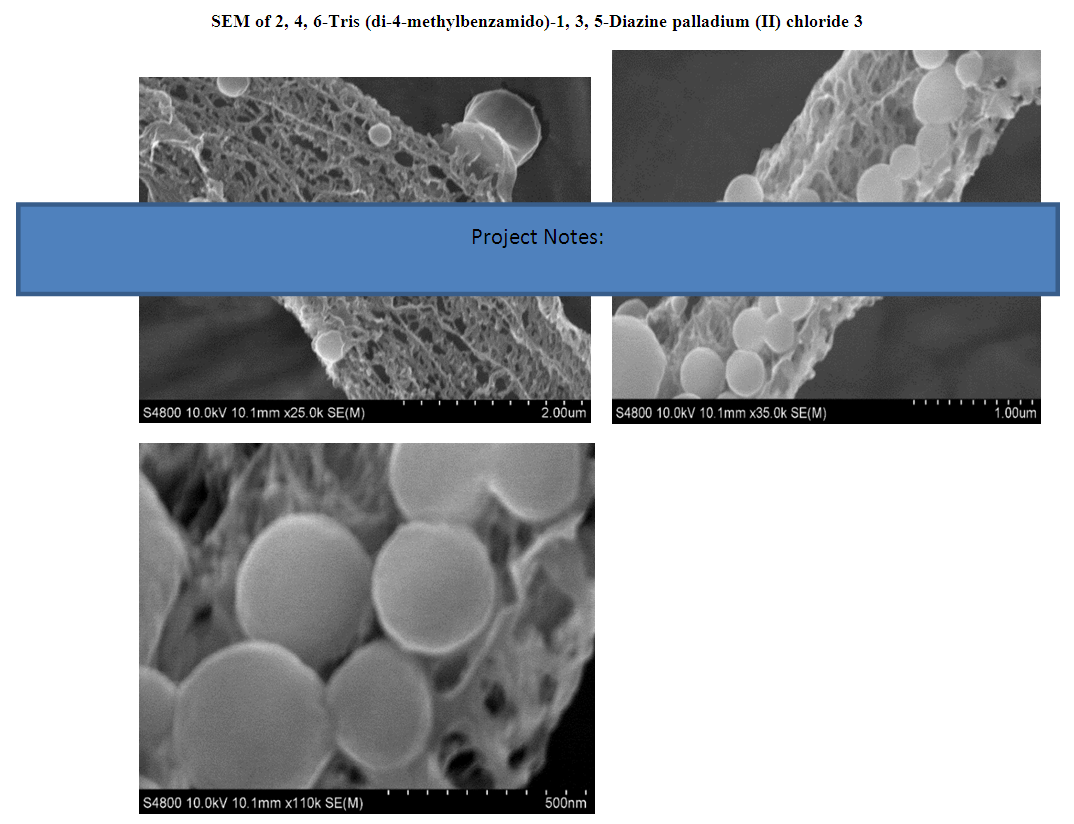
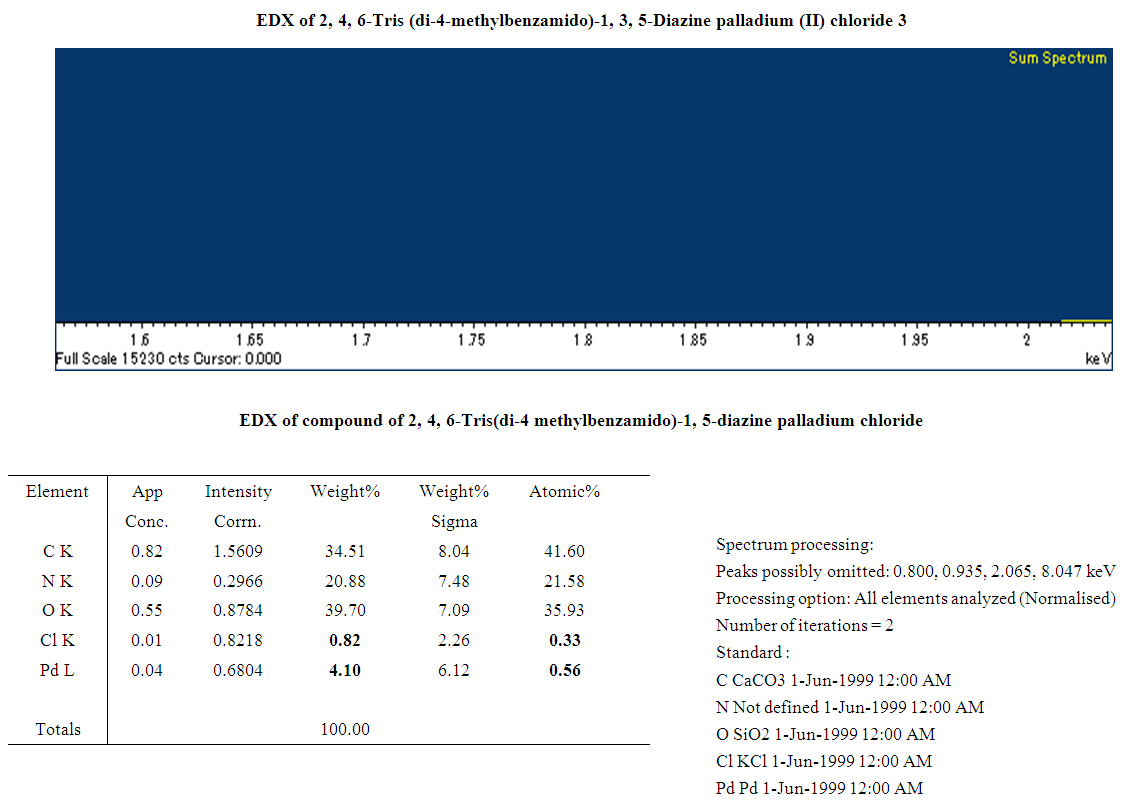
A palladium mediated metallodendrimer was synthesized via the reaction of 2,4,6-Triaminopyrimidine with 4-methyl benzoyl chloride using (Ph3P)2PdCl2 in DMF at 70°C used as an active homogeneous catalyst for the Sonogashira and Heck cross-coupling reactions. The catalyst was characterized by IR, NMR, Mass, and Elemental analysis. This palladium encapsulated metallodendrimer catalyst was also found to be a white crystalline solid air-stable state and highly effective in the phosphine ligand-free conditions for the coupling reactions. The fibril surface morphology and the presence of Palladium chloride of the compound were confirmed by SEM and EDX respectively whereas the good thermal stability of the catalyst was exhibited by TG and DSC techniques.
Keywords: Sonogashira Reaction, Heck Reaction, Aroyl chloride, Triaminopyrimidine, Metallodendrimer
Cite this paper: Md. Sayedul Islam, Md. Wahab Khan, Synthesis and Catalytic Activity of Palladium Mediated Metallodendrimer for the Sonogashira and Heck Coupling Reactions, American Journal of Chemistry, Vol. 9 No. 2, 2019, pp. 71-90. doi: 10.5923/j.chemistry.20190902.03.
Article Outline
1. Introduction
- Previous few decades, transition metal mediated cross-coupling reactions have become an essential implementation in organic synthesis. Among the transition metals, palladium is the utmost metal in recent organic synthesis and broadly used for the extensive quantity of synthetic transformations mostly carbon-carbon cross -coupling reactions [1-4]. The enormous significance of C-C bond forming reactions has fortified the chemical community to search for immensely active and strong palladium catalysts, which should be flexible and efficient. In addition, Metallodendrimers are the unique class of synthetic macromolecules having extraordinarily branched, three-dimensional, nano scale-shaped with very low polydispersity and immoderate functionality [5]. The metallodendrimer generally contains three superb areas which show active center in the different catalytic system: (i) metal atom as the dendrimer center, (ii) metal atoms inside the dendrimer branches (iii) metal atoms within the periphery. As for example, metals able to coordinate with the poly(amido)amine(PAMAM) shape encompass amongst others Cu [6, 7], Au [8], Pd [9], Pt [10], Ag [11], Co [12] as well as bimetallic structures such as Pd-Au [13] and Pt-Ru [14]. Lately, metallodendrimers were broadly researched in various fields, which includes molecular light harvesting, catalysts, liquid crystals, molecular encapsulation, and drug delivery [15]. Most of the investigation was succeeded in the arena of catalysis where metals, for example, Cu (II), Rh (III), Ru (II), Pd (II), Fe (I) and Co (III) are utilized in the production of metallodendrimers. Moreover, chemoselective reactions [17], and azide-alkyne reactions are also carried out and catalyzed by metallodendrimer compounds [18, 19]. Despite the fact that the ordinary Pd catalyzed and Cu co-catalyzed Sonogashira reactions has some disadvantages. These include the usage of extremely overpriced Pd catalysts (occasionally essential in high loading), difficulties in convalescing these catalysts, excessive reaction temperatures, air sensitivity of transition metal complexes and the usage of phosphine ligand which is air sensitive. In addition, the formation of homocoupled products due to contact of the alkynes to oxidizing agents, Cu salts or air as for example Glaser coupling [20] or Hay coupling [21] are also weaknesses. However, to minimize these drawbacks we designed as well as synthesized solid crystalline air stable diazine based dendrimer assisted Pd-metallodendrimer catalyst. It has shown efficient catalytic activity under mild and phosphine-free conditions for the heck and Sonogashira reactions.
2. Experimental Sections
- All procedures of air- and moisture-sensitive compounds were performed by the use of standard Schlenk techniques under an atmosphere of argon or nitrogen. The reagents were bought from Aldrich as high-purity products and usually used as received. Dehydrated DMF, DMSO, CH3CN, and THF were used as reaction solvent. The IR spectra was taken on a Shimadzu FTIR 8400S Fourier remodel Infrared Spectrophotometer (400-4000 cm-1) with KBr pellets. 1H NMR and 13C NMR spectra were recorded at 300 MHz and 75 MHz, respectively, on a JEOL AL 300/BZ tool in addition to BRUKER DPX-400 MHz & 100 MHz spectrophoto meters respectively. Chemical shifts was taken relative to TMS. Mass spectra (MS) was measured with the aid of the usage of AXIMA-CFR, Shimadzu/Kratos TOF Mass spectrometer. Scanning Electron Microscope (SEM) and power Dispersive X-ray (EDX) was taken on a Hitachi S-4800. Analytical thin layer chromatography (TLC) became silica gel 60 F 254 covered on 25 TCC aluminum sheets (20 × 20 cm). 2, 4, 6-triamino-1, 3, 5-diazine, 4-methyl benzoyl chloride and (Ph3P)2PdCl2 were purchased from Sigma Aldrich and had been directly used without further purification. The thermal behavior of metallodendrimer was determined by a thermogravimetric analyzer (NETZSCH STA 449F3) from 26 to 600°C. Elemental analyses were carried out with a Fisons EA 1108 CHNS-O apparatus. All TG and DSC facts had been received under a nitrogen environment by the use of aluminum oxide crucible at the heating rate of 10 °k/min and at a flow rate of 40 and 60 ml/min.
2.1. Synthesis of 2, 4, 6-Tris (di-4-methylbenzamido)-1, 3, 5-Diazine Palladium (II) chloride (3)
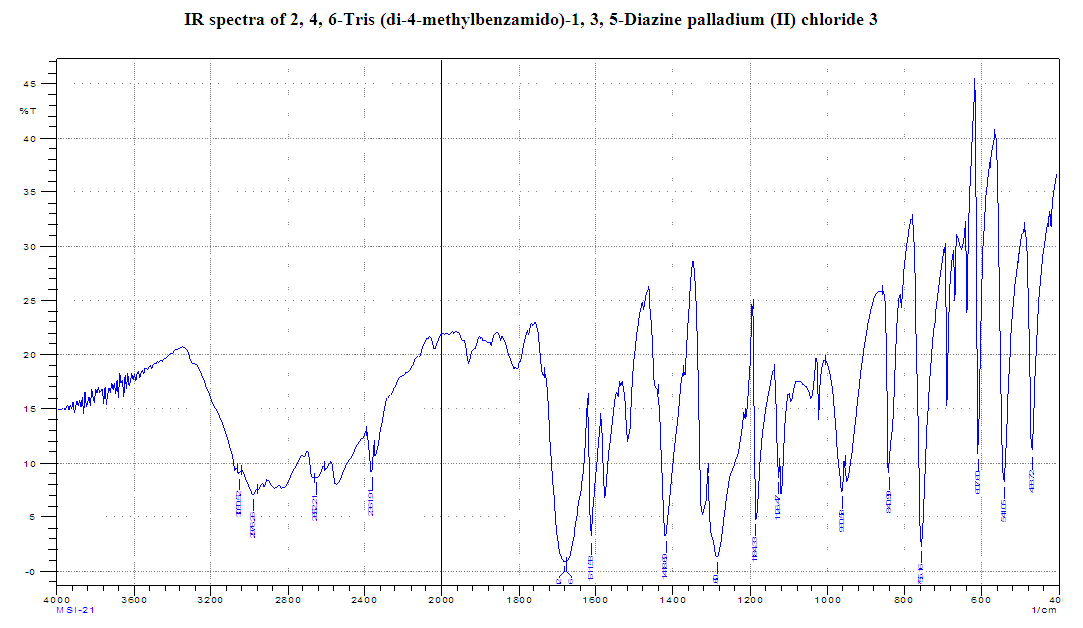
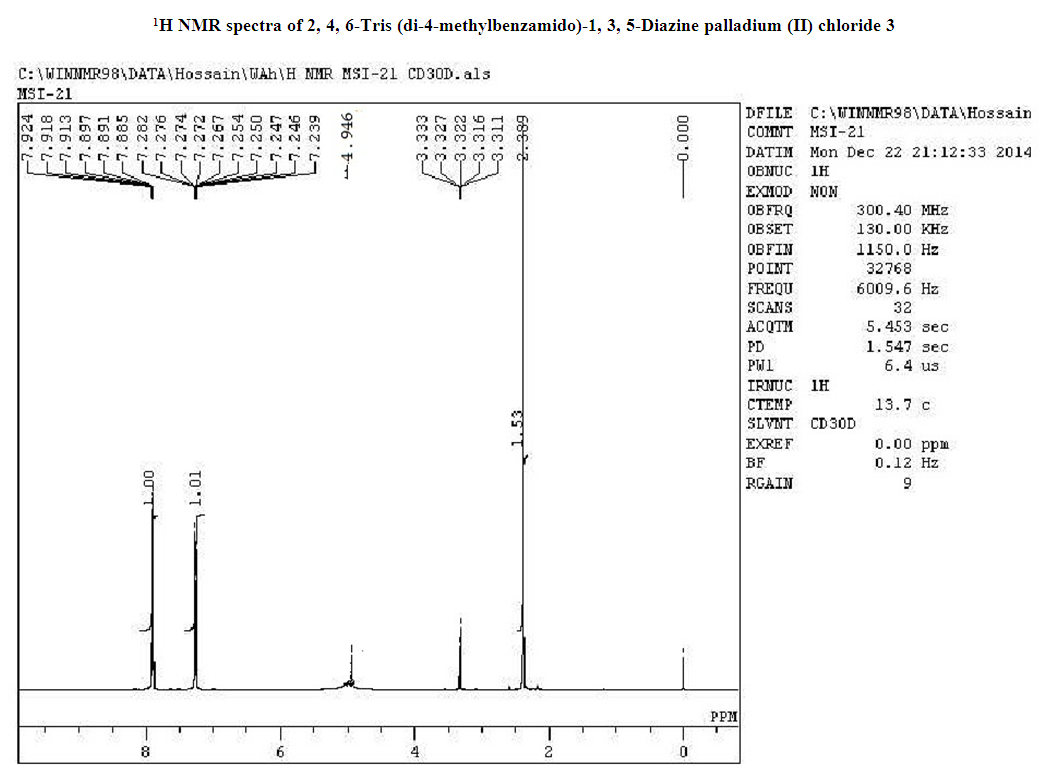
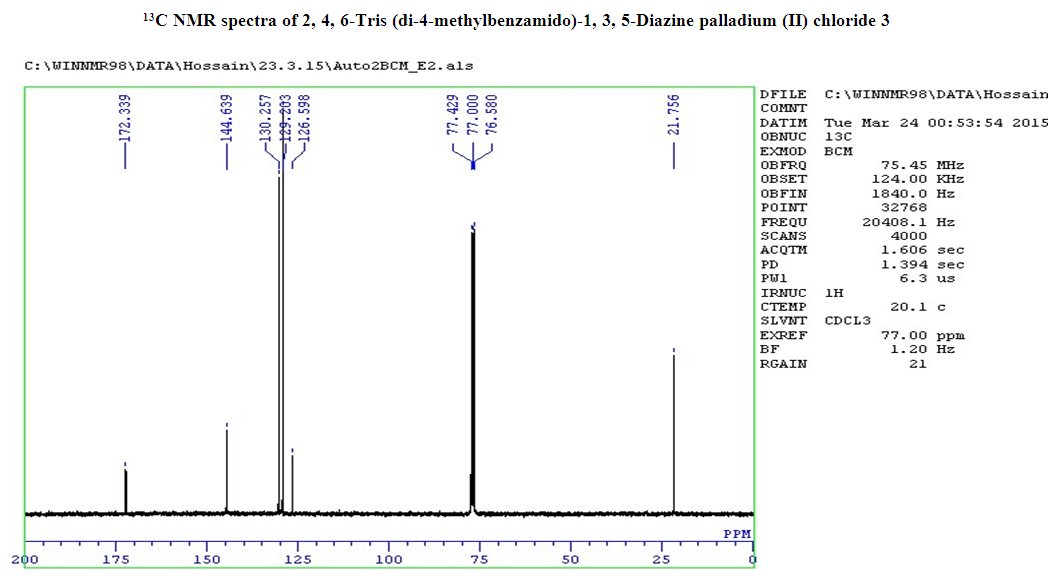
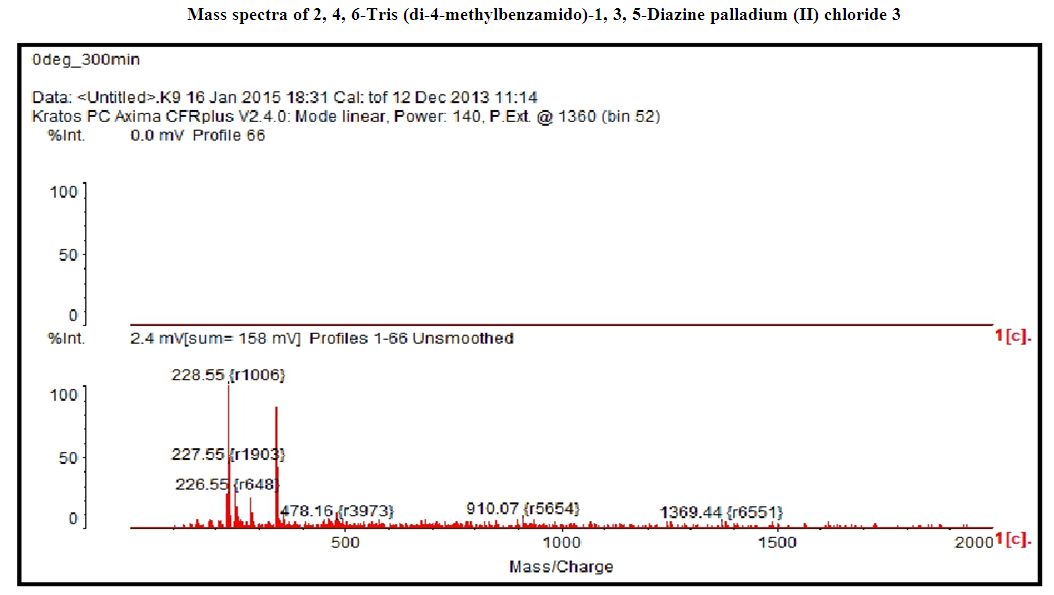
- 4-Methyl benzoyl chloride 2 (1.47 g, 9.54 mmol), (Ph3P)2PdCl2 (0.11g, 10% mol) were sequentially added to a solution of 2, 4, 6-triamino-1, 3, 5-diazine 1 (0.2 g, 1.59 mmol) in DMF (10 mL). The solution was degassed and stirred at room temperature under a nitrogen atmosphere for 1 h and the reaction was continued for 5 h at 70°C. The progress of the reaction was monitored by TLC. At the starting of the reaction, the mixture was a clear solution and after sometimes the reactants were turned into white solid precipitated and products was formed checked by TLC. After completion of the reaction, distilled H2O was added. After the removal of solvent, the product was washed with sodium hydrogen carbonate solution and purified by recrystallization by using ethanol and found the desired product 3. White crystalline solid; yield: 90%; IR (KBr): δ max 3052.29, 2976.31, 1710.20, 1611.69, 1418.69, 1360.20 cm-1.1H NMR (300 MHz, CD3OD): δ 7.80 (m, 12 H), 7.23 (m, 12 H), 4.96 (s, 1H) 2.3 (s, 18 H) ppm.13C NMR (75 MHz, CDCl3): δ 21.63, 129.28, 130.50, 131.28, 144.63 and 172.33 ppm. MALDI-TOF MS: m/z (%) = calcd. for C52N5H43O6 Pd3Cl6 1365.90; found 1369.44 (100) [M]+, Anal. Calcd. (%) for C52N5H43O6 Pd3Cl6: C, 45.70; H, 3.17; N, 5.13. Found: C, 45.67; H, 3.10; N, 5.10.
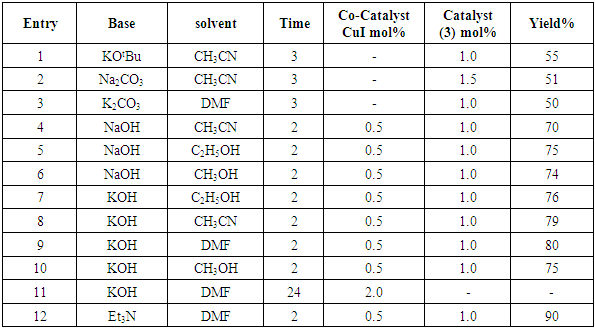
2.2. Catalytic Activity Tests: Palladium Mediated Metallodendrimer 3 in the Sonogasira Reaction
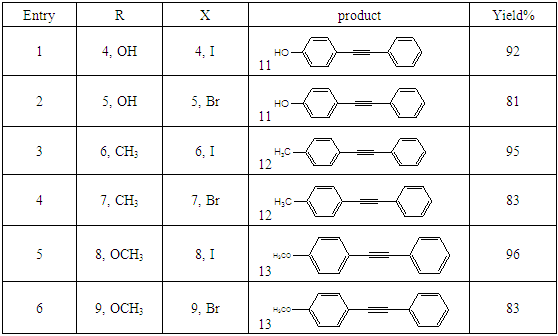
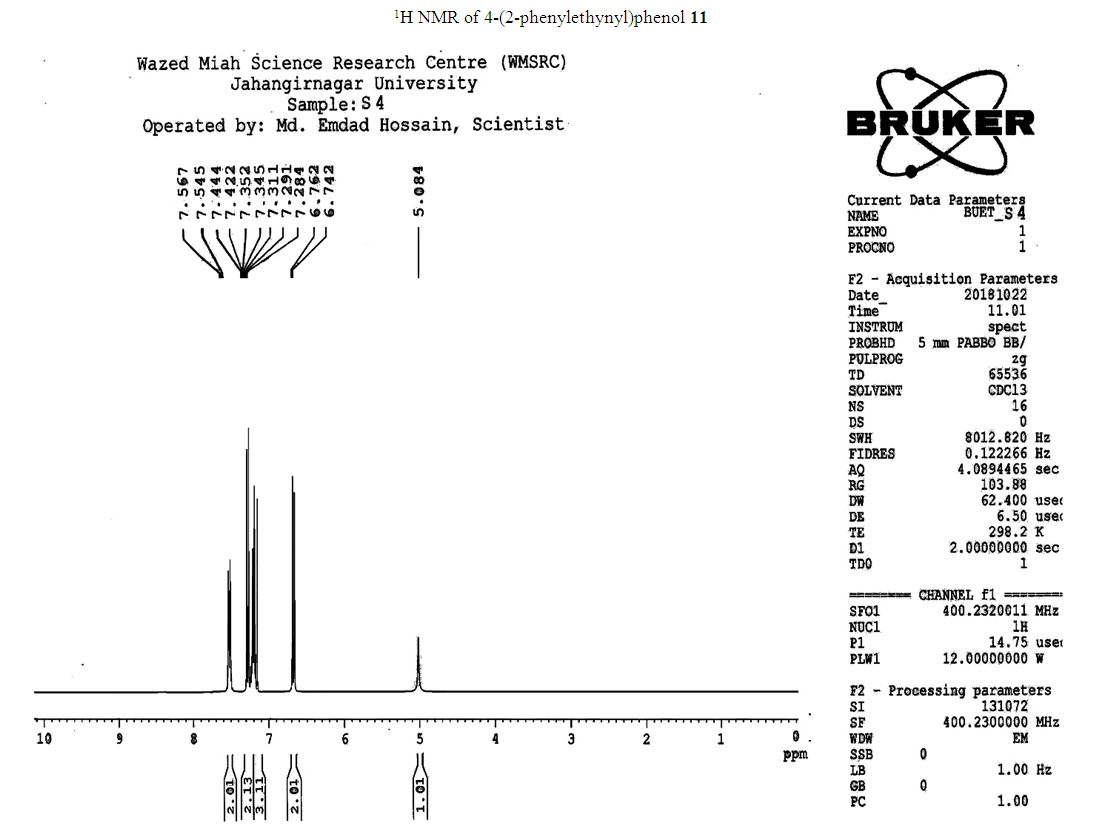
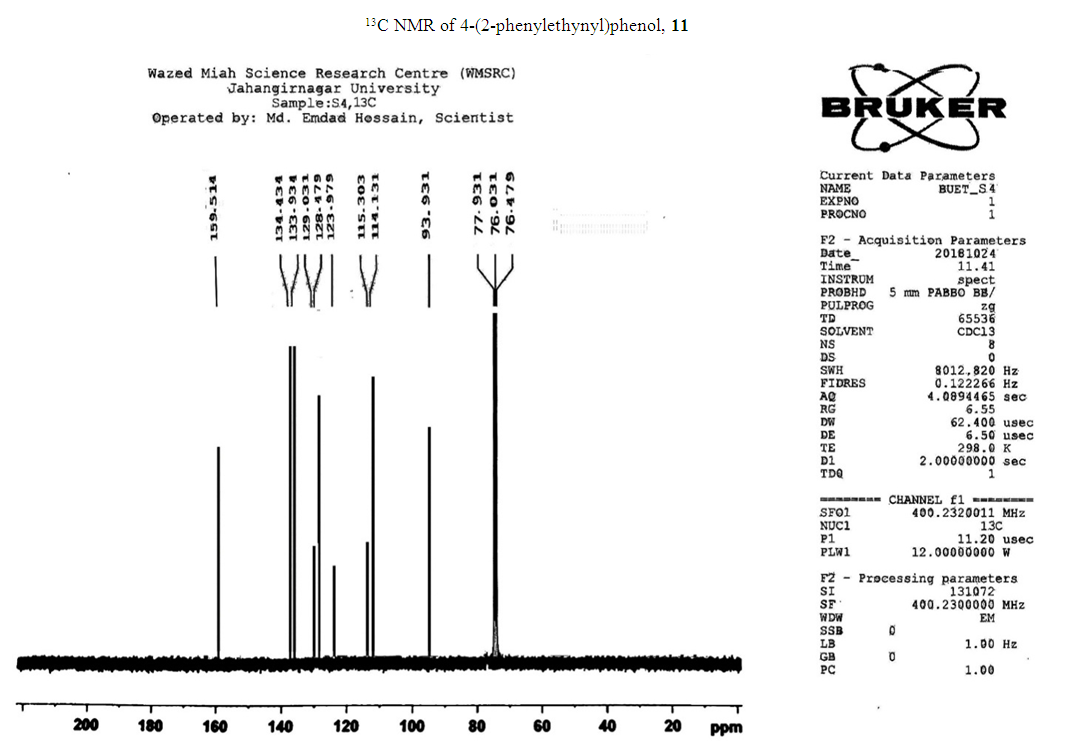
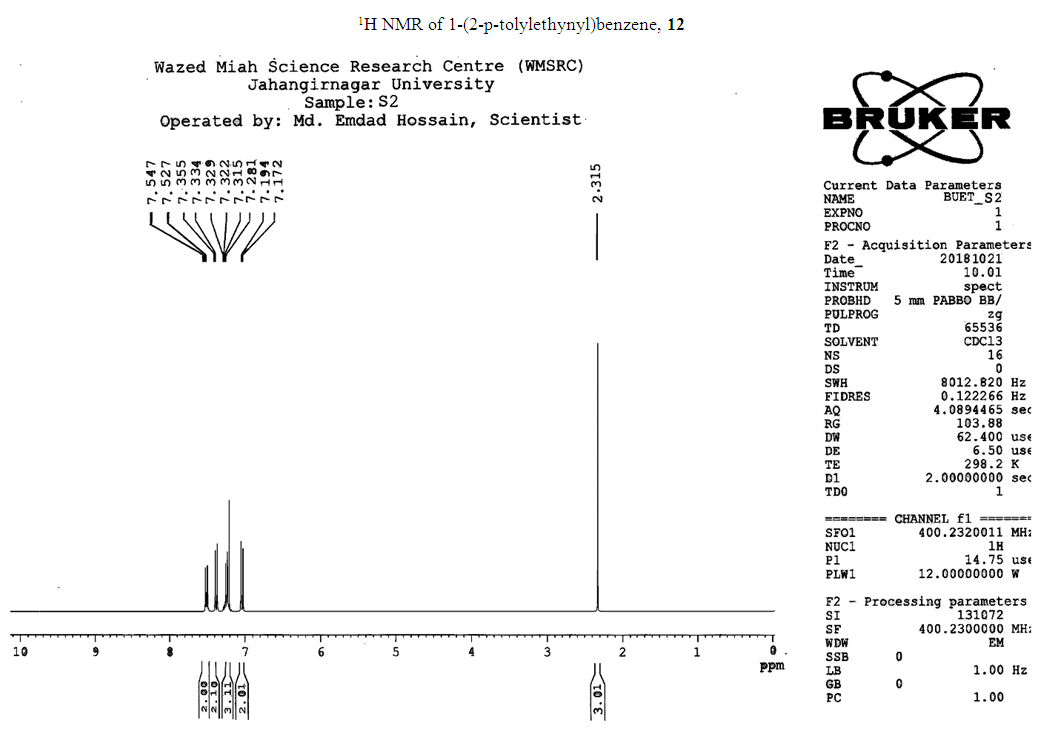
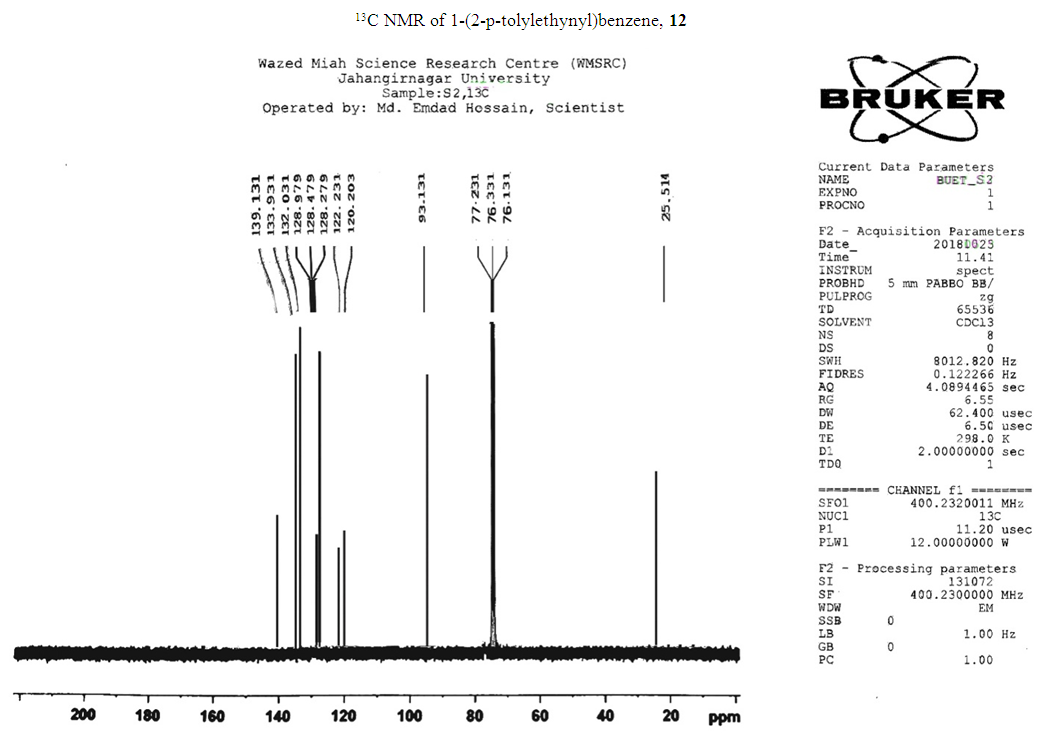
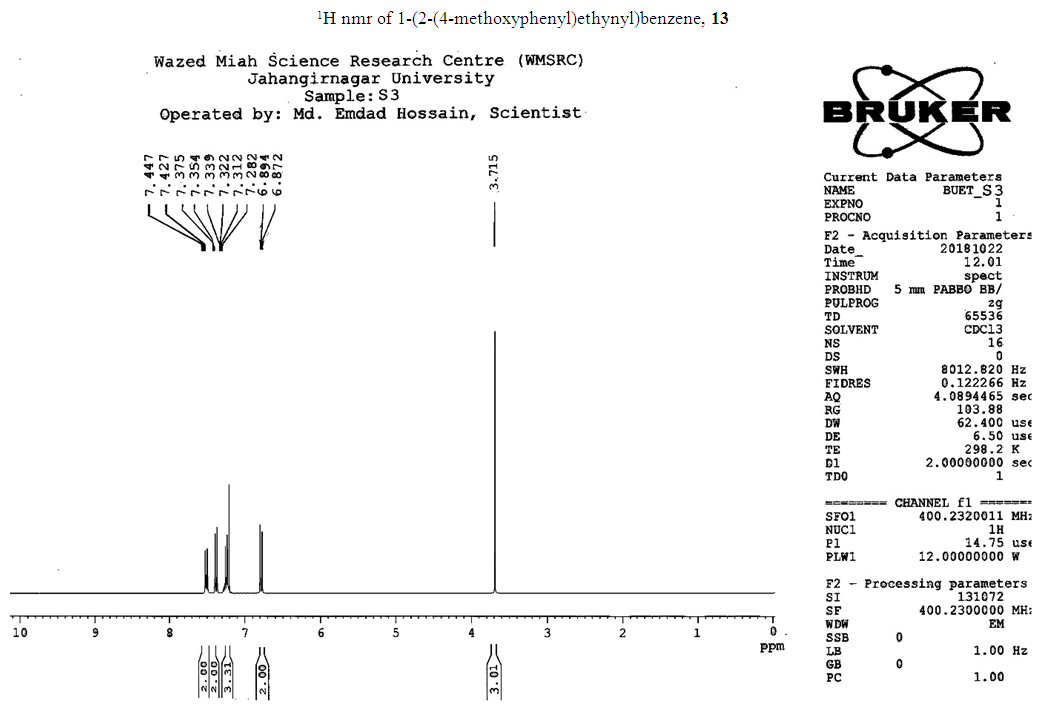
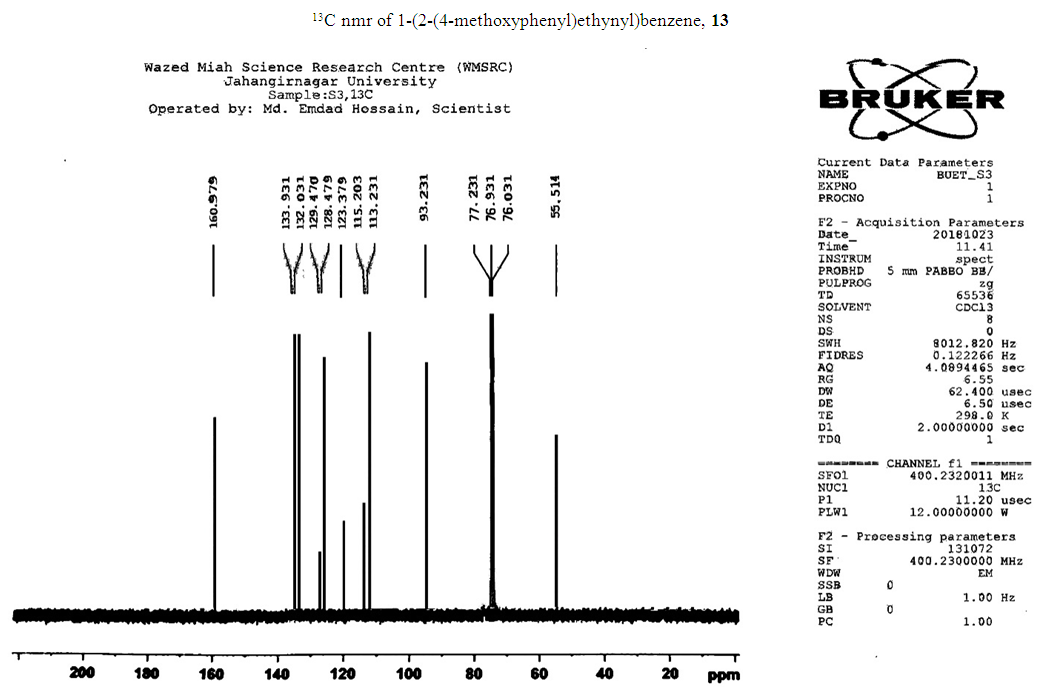
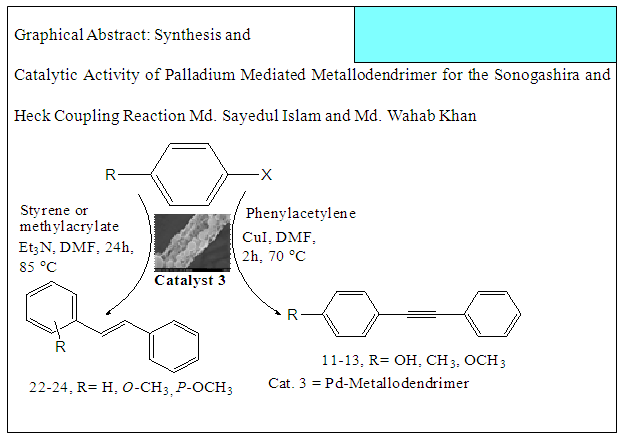
- Typical procedure: In a Schlenk tube equipped with a magnetic stirring bar were placed under nitrogen atmosphere with 1.0 mmol of aryl halide, (1.2 mmol) of phenylacetylene, 1 mol % of Pd-metallodendrimer 3, CuI 0.5 mol%, Et3N (2 mL) as a base and CH3CN (5 mL) as a solvent. The tube was degassed and the flask was immediately placed in an oil bath. The resulting mixture was stirred at 60°C temperatures for 3h and the reaction was checked by TLC. Then the solvent was vaporized under reduced pressure and the residue obtained was purified by silica gel chromatography using ethyl acetate and hexane (4:1).Synthesis of 4-(2-phenylethynyl)phenol 11, [22]Solid product was obtained, Yield 92%, mp: 124-126°C; 1H NMR (CDCl3, 400 MHz): δ 5.08 (s, 1 H, OH); 6.75 (d, 2H, J=8.8 Hz); 7.29-7.35 (m, 3 H); 7.43 (d, J= 8.8, 2H); 7.55 (d, J=8.8, 2H). 13C NMR (100 MHz, CDCl3): δ 93.93, 114.13, 115.30, 123.97, 128.47, 129.03, 133.93, 134.43, 159.51.Synthesis of 1-(2-p-tolylethynyl)benzene 12, [22]Solid colourless product was obtained, Yield 95%, mp: 70-72°C (lit. 71°C); 1H NMR (400 MHz): δ 2.31(s, 3 H); 7.20 (d, 2H, J= 8.8 Hz); 7.32 (t, 3H, J= 5.6 Hz); 7.33 (d, 2H, J=8.4 Hz); 7.54 (d, 2H, J= 8.0 Hz). 13C NMR (100 MHz, CDCl3): δ 92.51, 93.13, 120.20, 122.23, 128.27, 128.47, 128.97, 132.03, 133.93, 139.13.Synthesis of 1-(2-(4-methoxyphenyl)ethynyl)benzene 13, [23]Solid white product was obtained, Yield 96%, mp: 54-56°C (lit. 57°C); 1H NMR (400 MHz): δ 3.71 (s, 3H); 6.88 (d, 2H, J=8.8 Hz); 7.33 (t, J=10.8 Hz, 3H); 7.36 (d, 2 H, J=8.8 Hz); 7.44 (d, 2H, J=8.0 Hz), 13C NMR (100 MHz, CDCl3): δ 55.51, 93.23, 113.23, 115.20, 123.37, 128.47, 129.47, 132.03, 133.93, 160.97.
2.3. Catalytic Activity Tests: Palladium Mediated Metallodendrimer 3 in the Heck Reaction
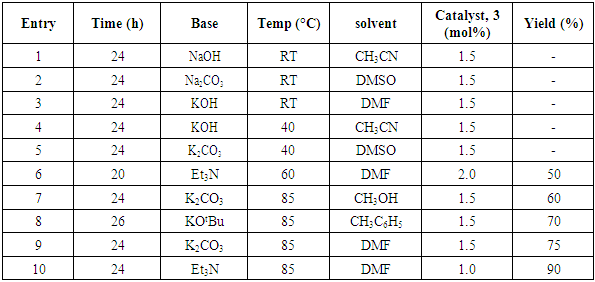
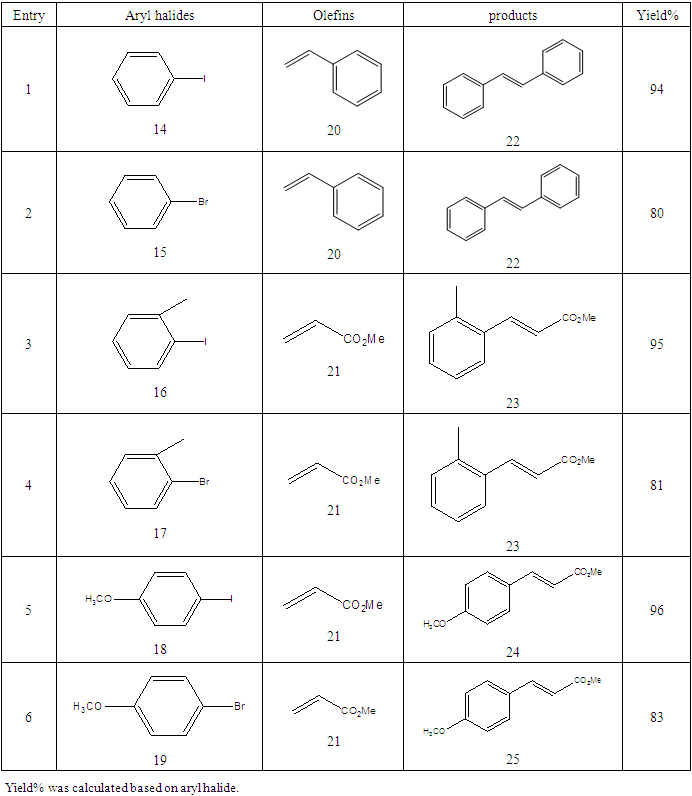
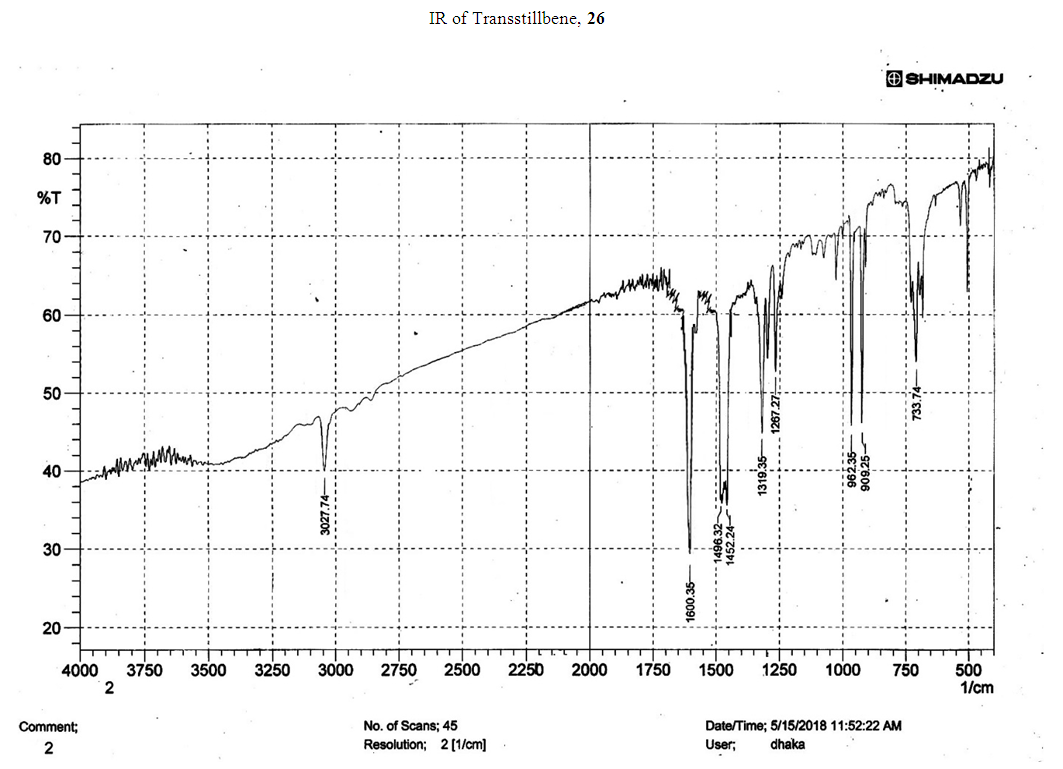
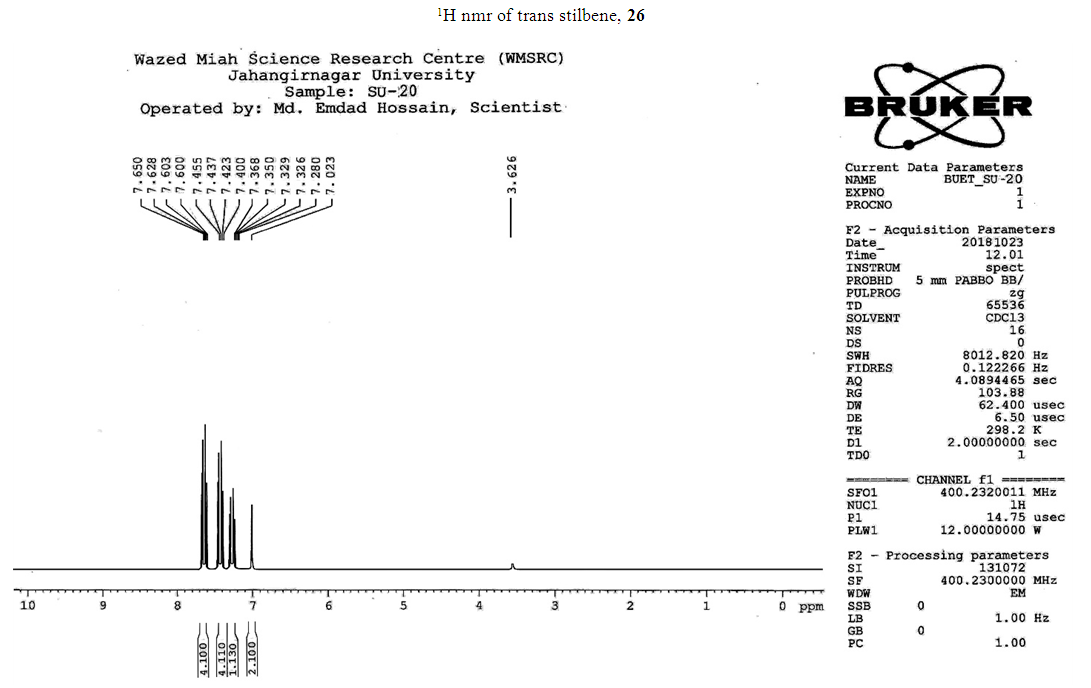
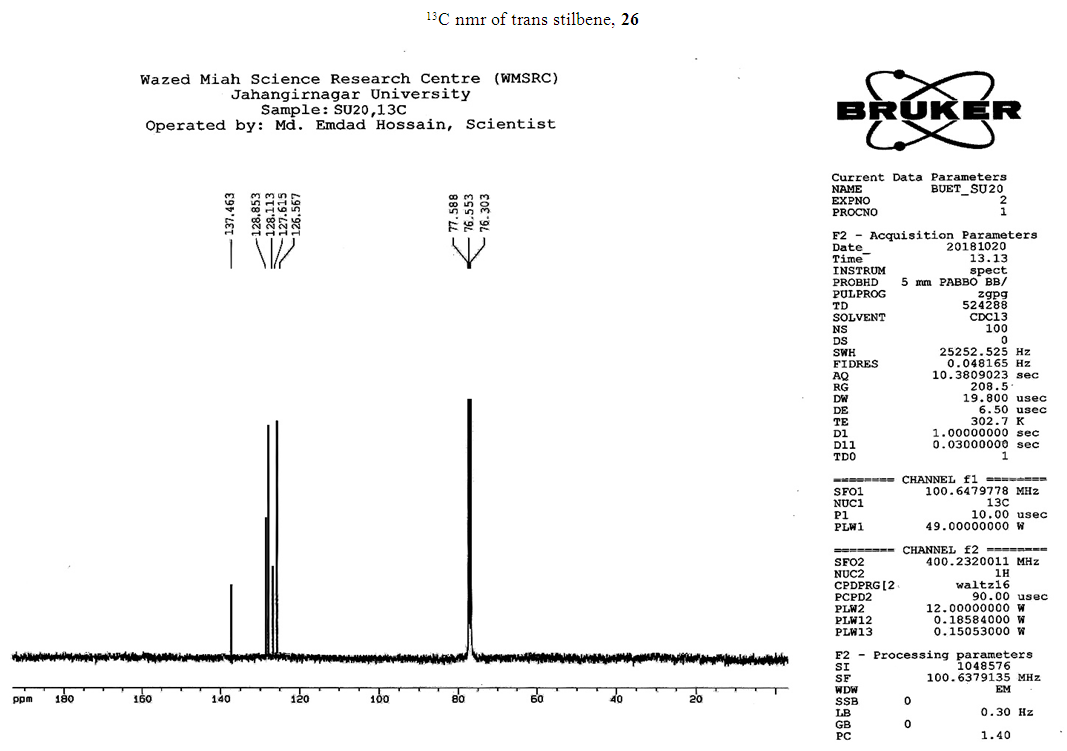
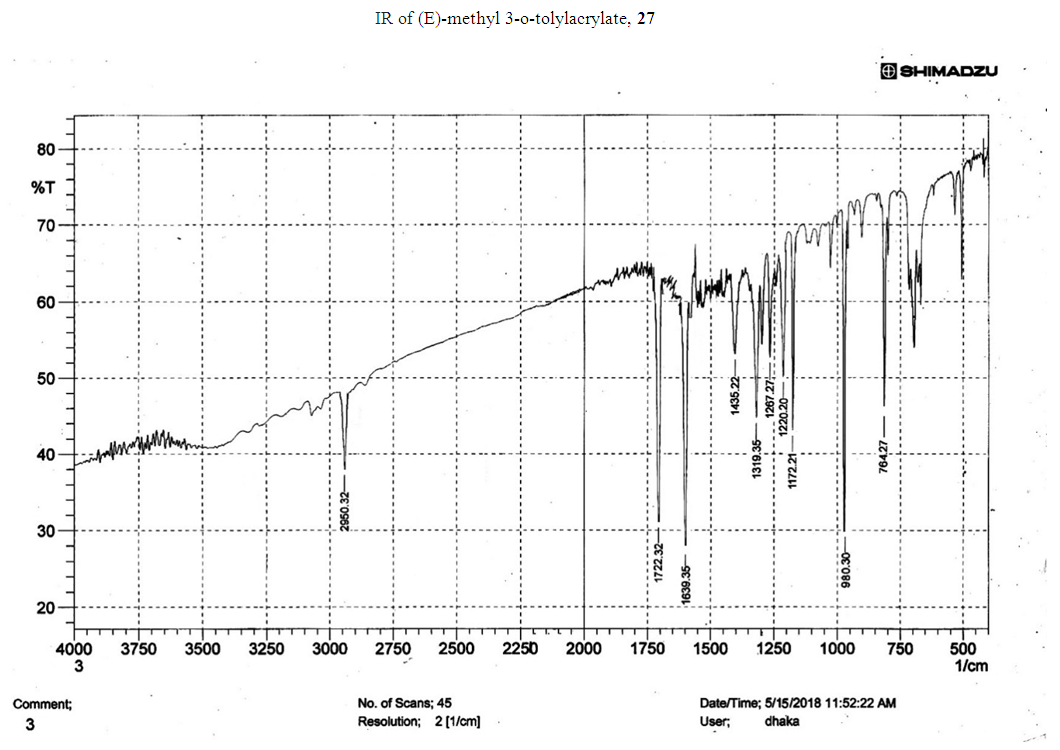
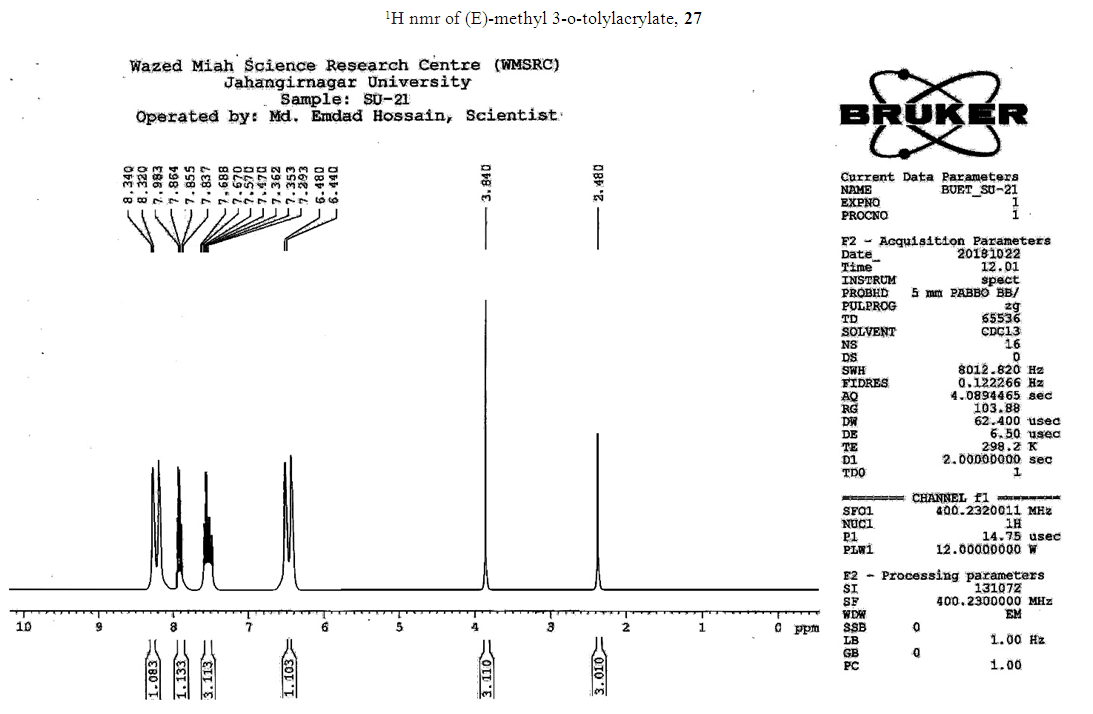
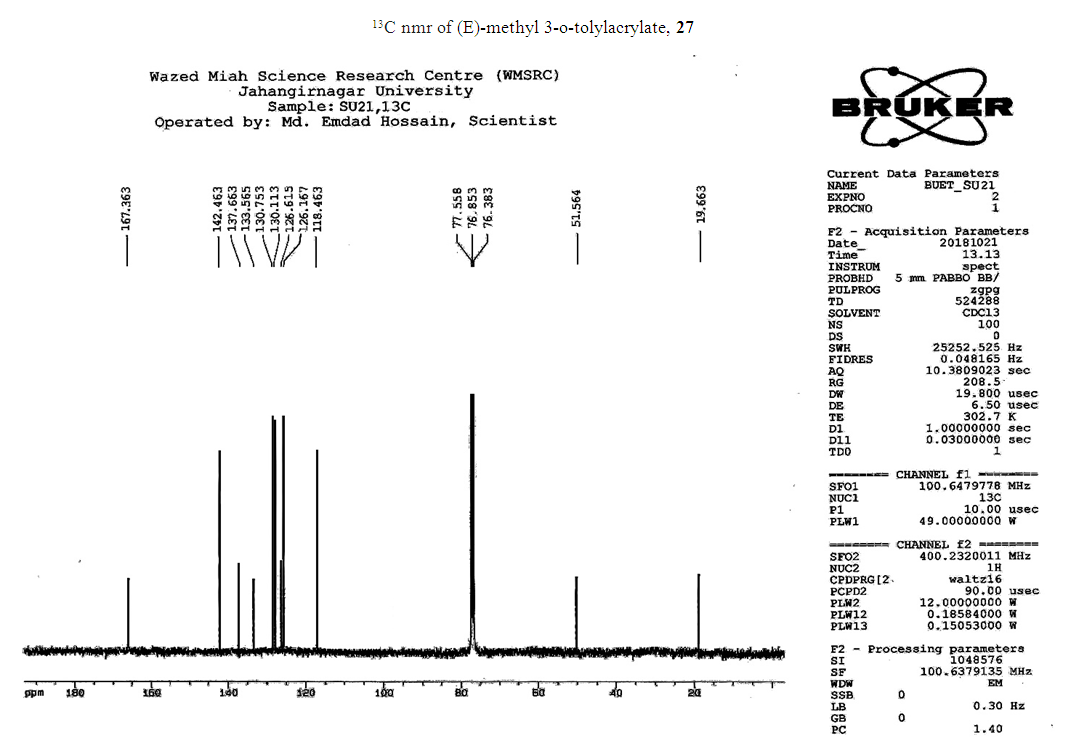
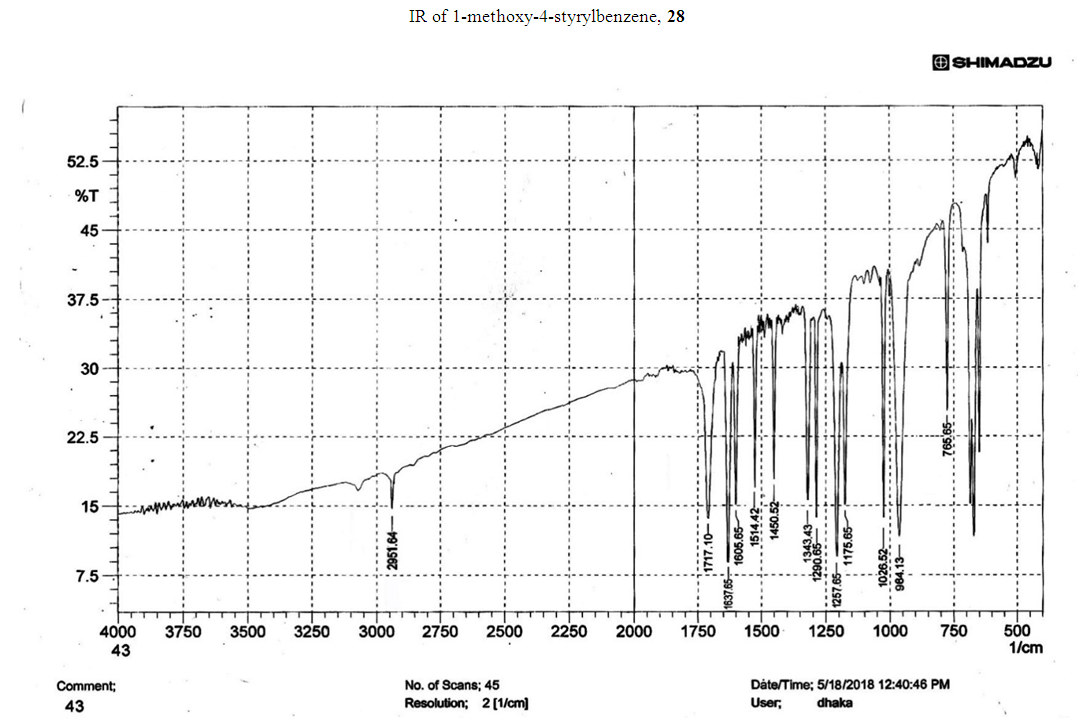
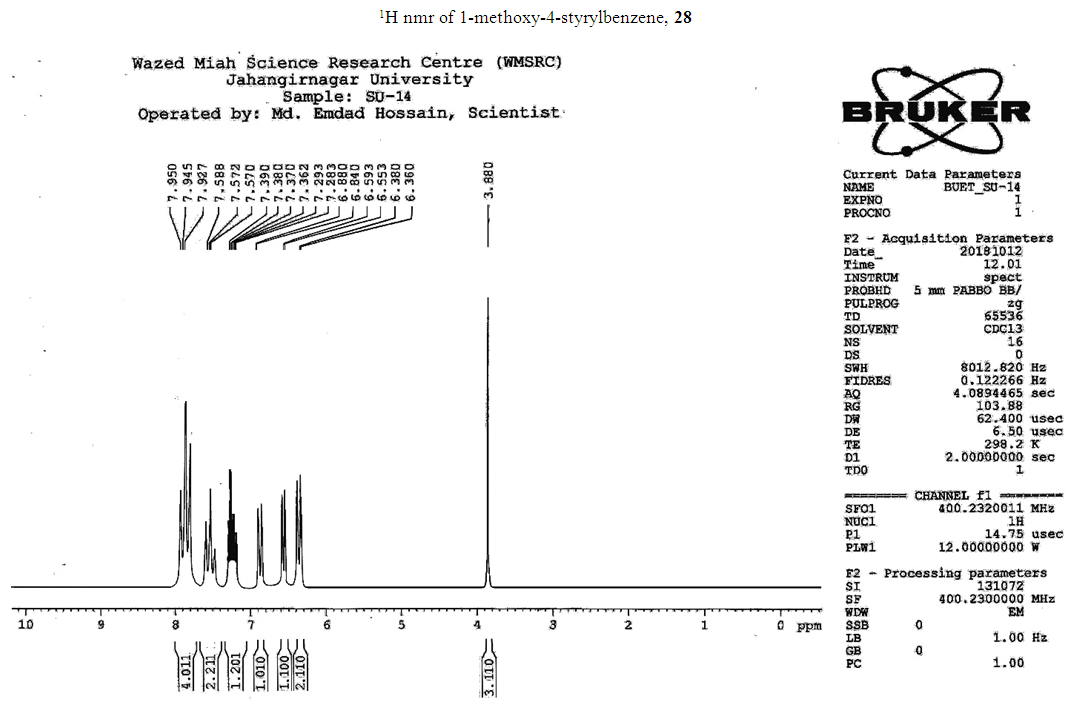
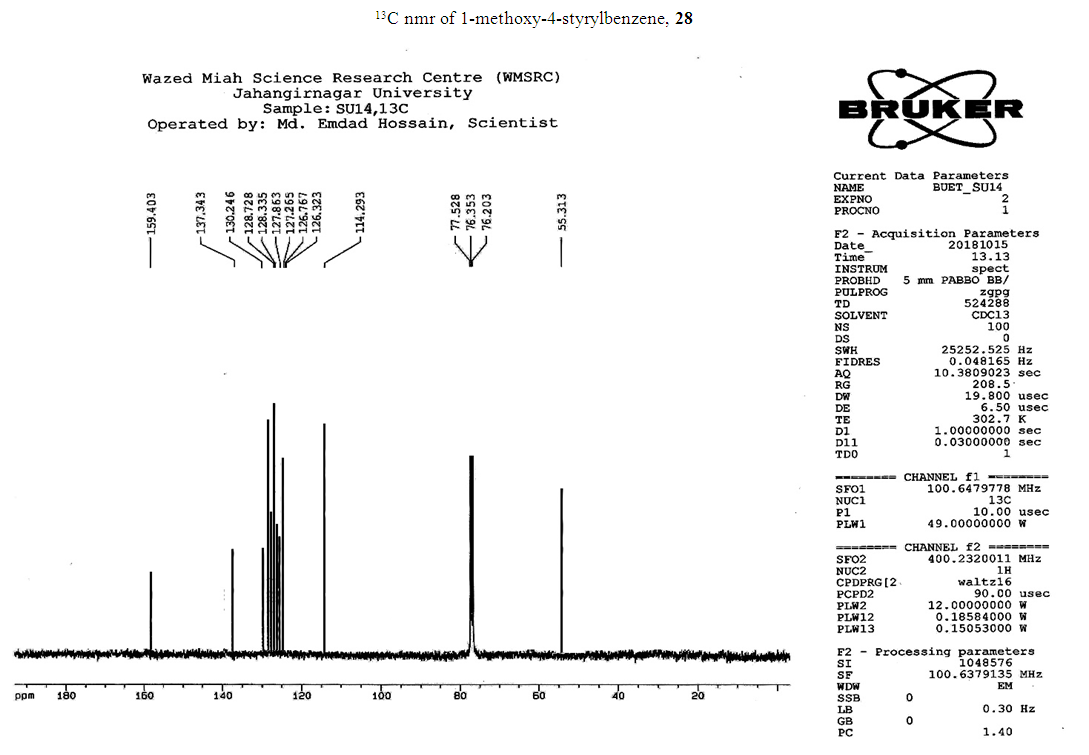
- Typical procedure: A combination of 4-iododophenol (1 mmol) with styrene (1.2 mmol), Pd-metallodendrimer 3 (1.0 mol%) and triethylamine (1.2 mL) was stirred in DMF (5 mL) in an R.B flask under nitrogen environment. The solution was heated at 85°C for 24 hrs. The advancement of the reaction was observed with the aid of TLC (n-hexane/ethyl acetate 1:1). After the achievement of the desired conversion of the reaction, the reaction mixture was evaporated to dryness under reduced pressure and the residue was turned into extracted with chloroform. The chloroform extract was washed with distilled water and dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. Then it was refined by silica gel column chromatography with n-hexane / ethyl acetate (3:1).Synthesis of trans stilbene, 22 [24] Colourless Solid was found and melting point was 74-76°C, Yield 94%, IR (KBr): δ max 3027, 1600.35, 1496, 1452.24, 1319.36, 1267.27, 962.35, 909.25, 733.74. 1H NMR (400 MHz, CDCl3), δ 7.02 (s, 2 H), 7.35 (dd, 1 H, J=1.2 Hz, 7.2 Hz), 7.43 (dd, 4 H, J=9.2 Hz, 7.2 Hz), 7.63 (dd, 4H, J=1.2 Hz, 8.8 Hz). 13C NMR (100 MHz, CDCl3): δ 126.56, 127.61, 128.11, 128.85, 137.46.Synthesis of (E)-Methyl 3-o-tolylacrylate 23, [24]Yield 95%, IR (KBr): δ max 2950.32, 1722.32, 1639.35, 1267.27, 1220.20, 1172.21, 980.30, 764.27. 1H NMR (400 MHz, CDCl3), δ 2.48 (s, 3H); 3.84 (s, 3H); 6.46 (d, 1H, J=16.0 Hz); 7.35-7.68 (m, 3H); 7.83-7.98 (m, 1H); 8.33 (d, J=8.0 Hz, 1H). 13C NMR (100 MHz, CDCl3): δ 19.66, 51.56, 118.46, 126.17, 126.61, 130.11, 130.75, 133.56, 137.66, 142.46, 167.36.Synthesis of 1-Methoxy-4-styrylbenzene 24, [24]White solid product was found, Yield 96%, mp. 135-137°C, IR (KBr): δ max 2960.74, 1601.35, 1511.24, 1319.35, 1251.27, 1179.75, 1031.15, 966.36, 812.25. 1H NMR (400 MHz, CDCl3), δ 3.88 (s, 3H); 6.37 (d, 2H, J=8.0 Hz); 6.57 (d, 1H, J=16 Hz); 6.86 (d, 1 H, J=16 Hz); 6.39-6.29 (m, 1H); 7.58 (t, 2H, J=7.2 Hz); 7.94 (t, 4 H, J=9.2 Hz). 13C NMR (100 MHz, CDCl3): δ 55.31, 114.29, 126.32, 126.76, 127.26, 127.86, 128.33, 128.77, 130.22, 137.34, 159.40.
3. Results and Discussion
- Metallodendrimer 3 was synthesized by the reaction of 2,4,6-Triaminopyrimidine 1(1.59 mmol) with 4-methyl benzoyl chloride 2 (9.54 mmol) in the presence of (Ph3P)2PdCl2 (10 mol%) in anhydrous DMF at room temperature to 70°C for 6 h under a nitrogen atmosphere (Scheme 1). The progress of the reaction was monitored by thin layer chromatography (TLC) and after complete conversion of the reaction, purification of the solid reaction mixture by recrystallization gave the dendrimerized product 3 in 92% yield (Table 1, entry 1). PdCl2 was also effective and provided 3 in 65% (entry 2). Since (Ph3P)2PdCl2 was given more yield than PdCl2, we selected (Ph3P)2PdCl2 for the later optimization. The use of tetrahydrofuran as the solvent caused in low yields of 3, because of the poor solubility of 3 (entry 3). DMSO also good solvent and gave 3 in 87% (entry 4). Increasing the amount of (Ph3P)2PdCl2 from 10 to 20 mol% did not progress the final yield of 3, the yield reduced due to the creation of a complex as a byproduct instead of the desired product (entry 5). The product was found to be soluble in all common organic solvents and was characterized by IR, 1H NMR, 13C NMR, and mass spectrometry.
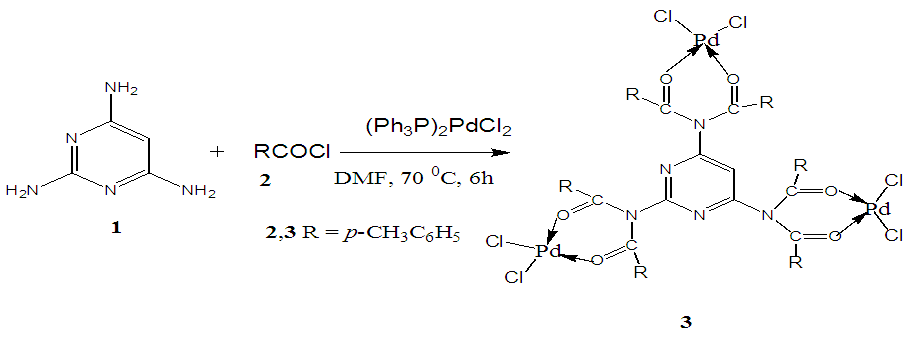 | Scheme 1 |
|
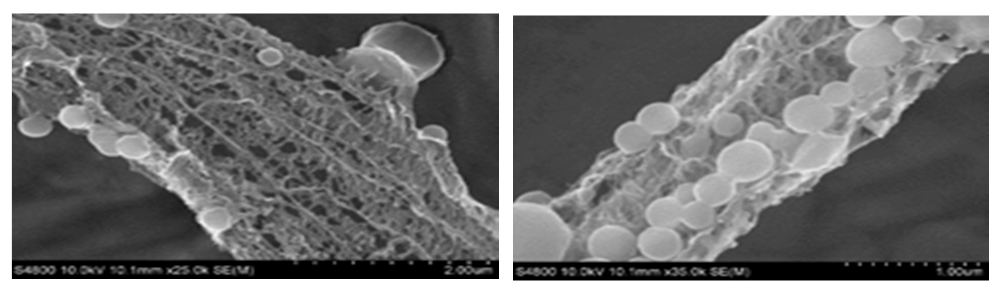 | Figure 1. SEM images of the compound 3 |
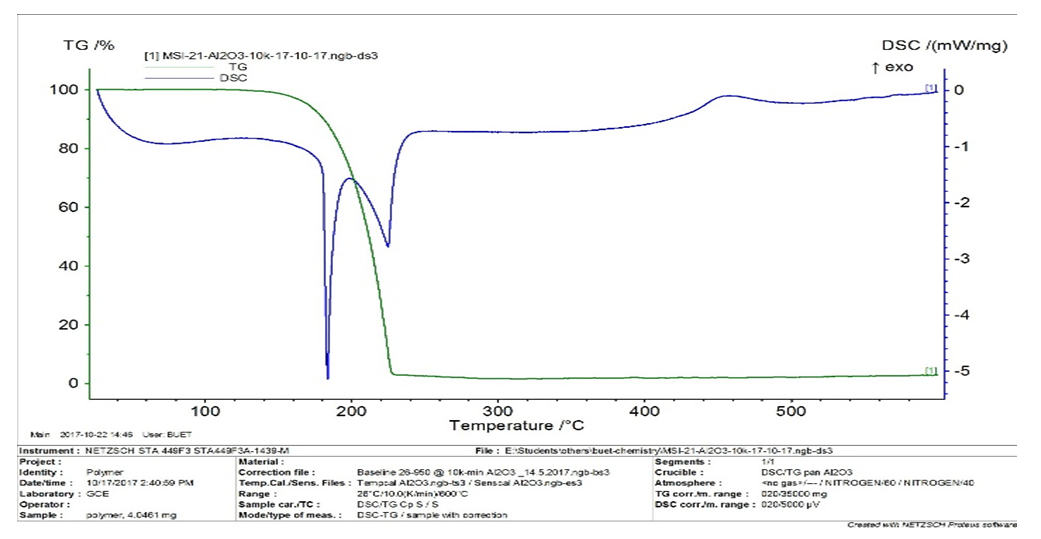 | Figure 2. TG and DSC curves of the compound 3 |
 | Scheme 2 |
|
 | Scheme 3 |
|
 | Scheme 4 |
|
|
4. Conclusions
- In precis, a unique palladium mediated metallodendrimer 3 was synthesized through the coordination reaction of 2,4,6-triaminopyrimidine (1) with 4-methyl benzoyl chloride (2). The complexation was virtually determined by IR, 1H NMR, 13C NMR, elemental analysis and mass spectra. The morphological structure of the catalyst like as branches of the tree without leaves or cylindrical fiber was revealed by SEM image and good thermal stability of the compound was observed by TG and DSC investigation. This homogeneous catalytic system showed numerous advantages including low catalyst loading, substrate tolerance, excellent yields, green solvents, short reaction times and a quite simple process for synthesizing Heck and Sonogashira coupling reaction products that are biologically vital in the numerous area in the chemistry world. These special upshots of this catalyst signify a noteworthy improvement in the area of C-C bond formation reactions.
ACKNOWLEDGEMENTS
- I would like to express our cordial gratefulness to the Ministry of Science and Technology, Dhaka, Bangladesh (National Science& Technology Ph.D Fellowship program 2018-2019, No- 39.00.0000.012.002.03.18.25, Code no-1260101-120005100-3821117) and Bangladesh University of Engineering and Technology (BUET), Dhaka, Bangladesh for funding the economic support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML