| [1] | Belyakova, O. A.; Slovokhotova, Y. L. (2003). Structures of large transition metal clusters. Russian Chemical Bulletin. Inter. Ed., 52(11), 1-29. |
| [2] | Butcher, C.P. G., Dyson, P. J., Johnson, B. F. G., Khimyak, J, McIndoe, J.S. (2003). Fragmentation of Transition Metal Carbonyl Clusters Anions: Structural Insights from Mass Spectrometry. Chem. Eur. J., 9(4), 944-950. |
| [3] | Ciabatti, I. (2015). PhD Thesis. Homo- and Hetero-metal carbonyl Nanoclusters. |
| [4] | Gimeno, M. C. (2008). Modern Supramolecular Gold-Metal Interactive and Applications. Edited A. Laguna, 2008. Wiley-VCH, Weinheim. |
| [5] | Fehlner, T.P. and Halet, J-F, (2007). Molecular Clusters, Cambridge University Press, UK. |
| [6] | Goicoechea, J. M. & Sevov, C. V. (2006). Deltahedral Germanium Cluster: Insertion of Transition-Metal Atoms and Addition of Organometallic Fragments. J. Am. Chem. Soc., 128, 4155-4161. |
| [7] | Grimes, R. N. (2003). Synthesis and properties of linear, branched and cyclic metallacarborane oligomers, 75(9),1211-1218. |
| [8] | Hoffmann, R. (1982). Building Bridges Between Inorganic and Organic Chemistry. Angew. Chem. Int. Ed. Engl. 21, 711-724. |
| [9] | Housecroft, C.E., Sharpe, A. G., (2005). Inorganic Chemistry, 2nd Ed., Pearson, Prentice Hall, Harlow, England. |
| [10] | Huang, X., Zhao, J., Chen, Z., King, R. B. (2014). Design of Three-shell Icosahedral Matryoshka Clusters A@B12@A20. Scientific Reports, 4, 1-7. |
| [11] | Hughes, H. K., & Wade, K. (2000). Metal-metal and metal-ligand bond strengths in metal carbonyl clusters. Coord. Chem. Rev., 197, 191-229. |
| [12] | Jemmis, E. D., Balakrishnarajan, M.M., Pancharatna, P. D. (2001a). Unifying electron counting rule for Macropolyhedral Boranes, Metallaboranes, and Metallocenes. J. Am. Chem. Soc., 123(18), 4313-4323. |
| [13] | Jemmis, E. D., Balakrishnarajan, M. M. (2001b). Polyhedral boranes and elemental boron. Direct structural relations and diverse electronic requirements. J. Am. Chem. Soc., 123, 4324-4330. |
| [14] | Jemmis, E. D., Balakrishnarajan, M.M., Pancharatna, P. D. (2002). Electronic Requirements for Macropolyhedral Boranes. Chem. Rev. 102(1), 93-144. |
| [15] | Jemmis, E. D., Jayasree, E. G. (2003). Analogies between boron and carbon. Acc. Chem. Res., 36, 816-824. |
| [16] | Jemmis, E. D. (2005). Building relationships between polyhedral boranes and elemental boron. Inorg. Chem. 18, 620-628. |
| [17] | Jemmis, E. D., Jayasree, E. G., Parameswaran, P. (2006). Hypercarbons in polyhedral structures. Chem. Soc. Rev., 35, 157-168. |
| [18] | Jemmis, E. D., Prasad, D. L. V. K. (2008). Unknowns in the chemistry of Boron. Current Science, 95(10), 1277-1283. |
| [19] | King, R.B., Zhao, J. (2006). The isolable matryoshka nesting doll icosahedral cluster, As@Ni12@As203―, as a superatom: analogy with the jellium cluster Al13― generated in the gas phase by laser vaporization. Chem. Comm., 4204-4205. |
| [20] | Kiremire, E.M.R. (2015). A Uniquebypass to the Carbonyl Cluster Nucleus Using the 14N Rule. Orient.J. Chem.31(3),1469-1476. |
| [21] | Kiremire, E.M.R. (2016a). A Hypothetical Model for the Formation of Transition Metal Carbonyl Clusters Based upon 4n Series Skeletal Numbers. Int. J. Chem., 8(4), 78-110. |
| [22] | Kiremire, E. M. R. (2016b). The Application of the 4n Series Method to Categorize Metalloboranes. Int. J. Chem., 8(3), 62-73. |
| [23] | Kiremire, E. M. (2015d). Classification of Transition Metal Carbonyl Clusters Using the 14n Rule Derived from Number Theory. Orient. J. Chem., 31(2), 605-618. |
| [24] | Kiremire, E.M.R. (2016c). The categorization and Structural Prediction of Transition Metal Carbonyl Clusters Using the 14n Series Numerical Matrix. Int. J. Chem. 8(1), 109-125. |
| [25] | Kiremire, E. M. R. (2016b). A Hypothetical Model for the Formation of Transition Metal Carbonyl Clusters Based Upon 4n Series Skeletal Numbers. Int. J. Chem., 8(4), 78-110. |
| [26] | Kiremire, E. M. R. (2016c). The Application of the 4n Series Method to Categorize Metalloboranes. Int. J. Chem., 8(3), 62-73. |
| [27] | Kiremire, E.M.R. (2016d). Classification of Zintl Ion Clusters Using 4n Series Approach. Orient. J. Chem., 32(4), 1731-1738. |
| [28] | Kiremire, E. M. R. (2017a). The Six Silent Laws of Chemical Clusters. Amer. J. Chem. 7(2), 21-47. |
| [29] | Kiremire, E. M. R. (2017b). Outstanding Applications of Skeletal Numbers to Chemical Clusters. Int. J. Chem., 9(3), 28-48. |
| [30] | Kiremire, E. M.R. (2017c). Boranes, Carboranes, Metalloboranes, Transition Metal Carbonyls, and Other Cluster Formulas Obey the Law of Skeletal Numbers and Their Valences. Amer. J. Chem., 7(4), 113-144. |
| [31] | Kiremire, E. M.R. (2017d). Numerical Characterization of Chemical Fragments, Molecules, and Clusters Using Skeletal Numbers and Nuclearity Trees. Amer. J. Chem., 7(3), 73-96. |
| [32] | Kiremire, E.M.R. Kiremire (2017e). Numerical categorization of Chemical Fragments, Molecules and Clusters Using Skeletal Numbers and Nuclearity Trees. Am. J. Chem., 7(3), 73-96. |
| [33] | Kiremire, E. M. R. (2017f). The Golden Series and Clusters of Gold-unique Shapes and Bonding. 9(1), 38-57. |
| [34] | Kiremire, E.M.R. (2018a). The Cluster Valence Electrons (VE) are Natural Numbers of Clusters Generated by K(N) Parameters: VE and K(N) Are Intertwined. Int. J. Chem., 10(1), 15-52. |
| [35] | Kiremire, E.M.R. (2018b). The capping theory of chemical clusters based on 12N/14N Series, Int. J. Chem, 10(4), 130-154. |
| [36] | Kiremire, E.M.R. (2018c). Graph Theory of Chemical Series and Broad Categorization of Clusters. Int. J. Chem., 10(1), 17-80. |
| [37] | Kiremire, E.M.R. (2018d). Graph Theory of Capping Golden Clusters. Int. J. Chem., 10(1), 87-130. |
| [38] | Kiremire, E.M.R. (2018e). Inside out Capping Clusters: Matryoshka series. Int. J. Chem., 10(4), 38-56 |
| [39] | Kiremire, E.M.R. (2018f). Graph Theory of Chemical Series and Broad Categorization of Clusters. Int. J. Chem.,10(1),17-80. |
| [40] | Lipscomb, W. N., (1963). Boron Hydrides. W. A. Bejamin, Inc., New York. |
| [41] | Mednikov, E., Dahl, L. F. (2010). Syntheses, structures and properties of primarily nanosized homo/heterometallic palladium CO/PR3-ligated clusters. Phil. Trans. R. Soc., 368, 1301-1331. |
| [42] | Mingos, D. M. P. (1972). A General Theory for Cluster and Ring Compounds of the Main Group and Transition Elements. Nature (London), Phys. Sci., 236, 99-102. |
| [43] | Pauling, L. (1977). Structure of transition-metal cluster compounds: use of an additional orbital resulting from f, g character spd bond orbitals. Proc. Natl. Acad. Sci. USA, 74, 5235-5238. |
| [44] | Rossi, F., Zanello, P. (2011). Electron Reservoir Activity of High-Nuclearity Transition Metal Carbonyl Clusters. Portugaliae Electrochimica Acta, 29(5), 309-327. |
| [45] | Rudolph, R. W. (1976). Boranes and heteroboranes: a paradigm for the electron requirements of clusters? Acc. Chem. Res., 9(12), 446-452. |
| [46] | Slee, F, Zhenyang, L Mingos, D. M.P. (1989). Polyhedral Skeletal Electron Pair Theory of Bare Clusters: Small Silicon Clusters. Inorg. Chem., 2256-2261. |
| [47] | Stone, A (1981). New Approach to Bonding in Transition Metal Clusters and related compounds. 20, 563-571. |
| [48] | Teo, B. K., Longoni, G., & Chung, F.R. K. (1984). Applications of Topological Electron-Counting Theory to Polyhedral Metal Clusters. Inorg. Chem., 23(9), 1257-1266. |
| [49] | Tolman, C. A. (1972). The 16 and 18 Electron Rule in Organometallic Chemistry and Homogeneous catalysis. Chem. Soc. Rev., 337-353. |
| [50] | Wade, A. (1976). Structural and Bonding Patterns in Cluster Chemistry. Adv. Inorg. Chem. Radiochem., 18, 1-66. |
| [51] | Wade, K. (1971). The structural significance of the number of skeletal bonding electron-pairs in carboranes, the higher boranes and borane ions and various transition metal carbonyl cluster compounds. Chem. Commun., 792-793. |
| [52] | Wade, A. (1976). Structural and Bonding Patterns in Cluster Chemistry. Adv. Inorg. Chem. Radiochem., 18, 1-66. |
| [53] | Wales, D. J. (2005). Electronic Structure of Clusters in Encyclopedia of Inorganic Chemistry, 2nd Edition, Vol III. Edited, R. B. King, John Wiley and Sons, Ltd., Chichester, UK, 1506-1525. |
| [54] | Welch, A. J. (2013). The significance of Wade’s rules. Chem. Commun., 49, 3615-3616. |
| [55] | Xiang, W., Sikadar, D., Yap, L.W., Guo, P., Premaratne, M., Li, X., Cheng, W. (2015). Tsinghua University Press, 64-80. |

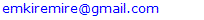

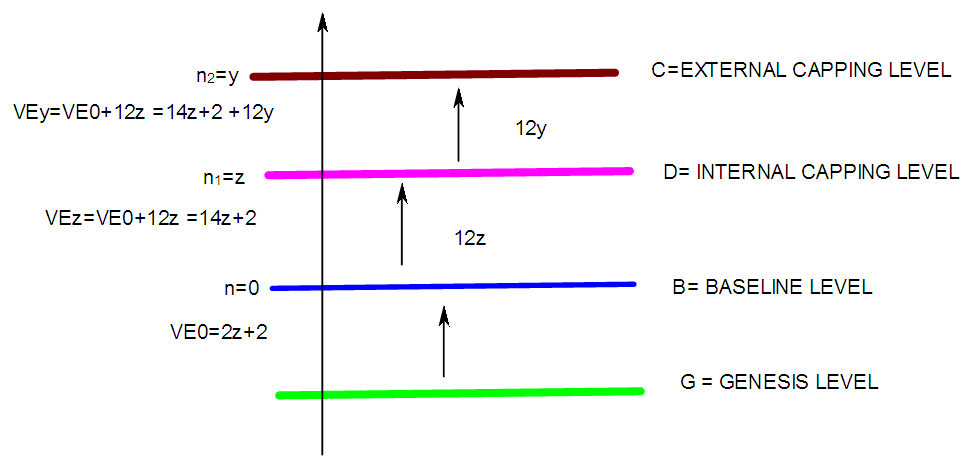
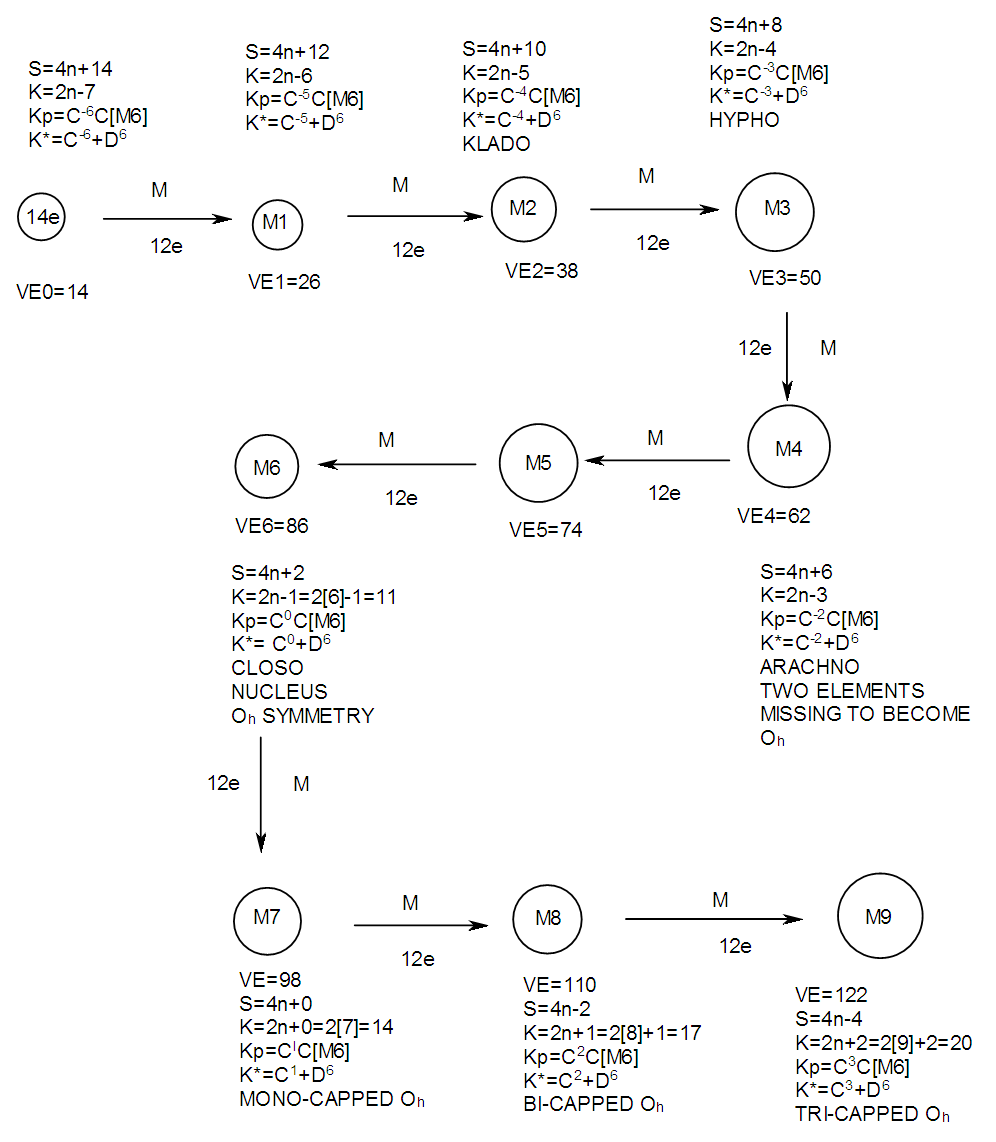
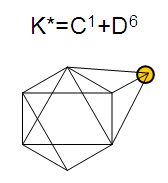
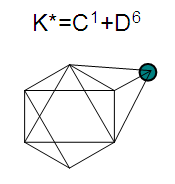
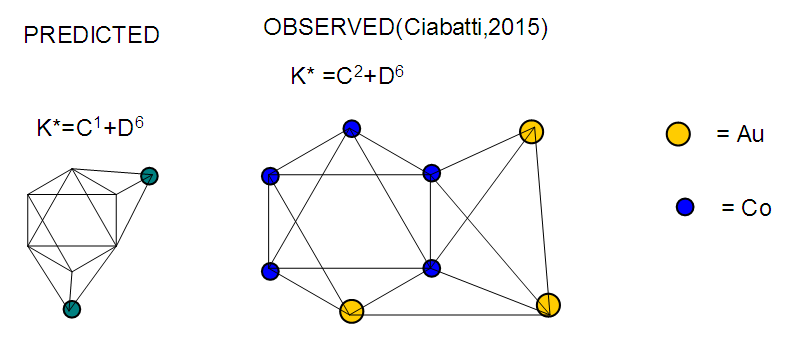
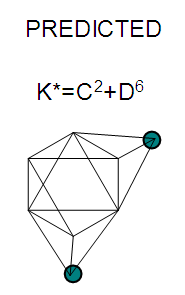
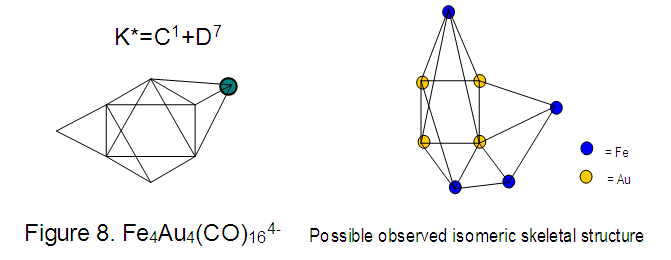
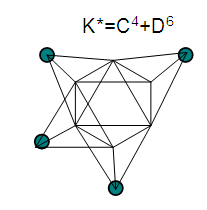
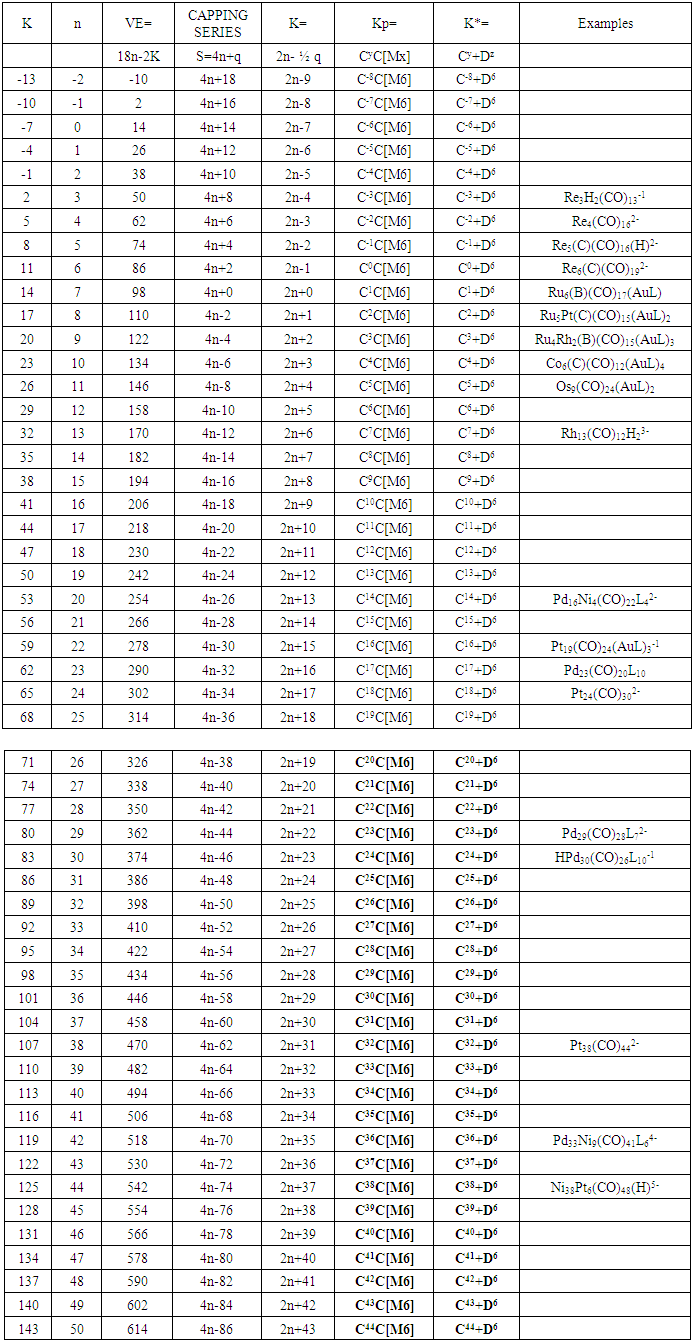
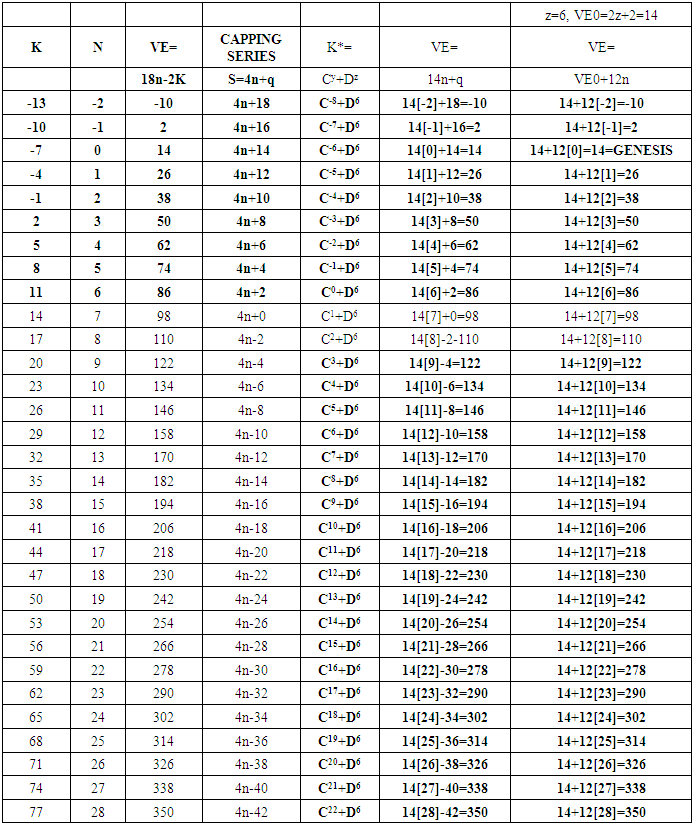
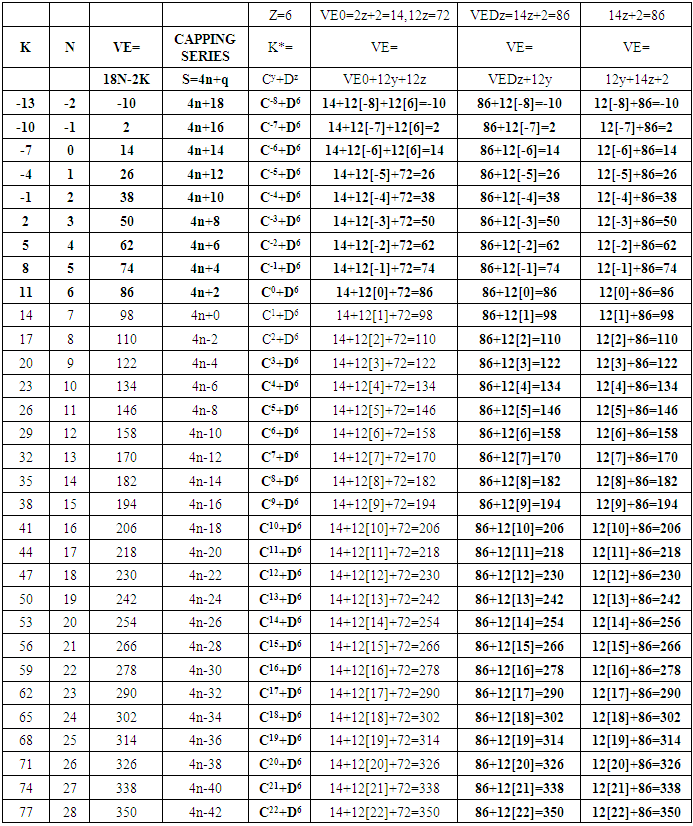
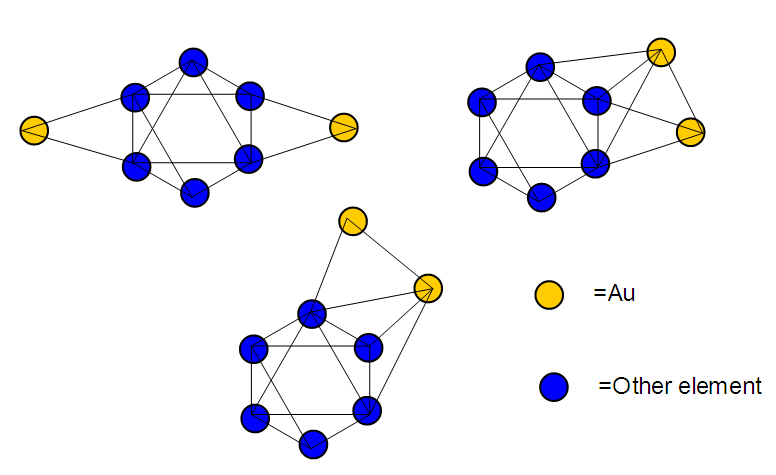
 17. Ir6Ru3(CO)21(AuL)-1:K=6[4.5]+3[5]-21[1]+1[3.5-1]-1[0.5]=23n=6+3+1=10K(n)=23(10)2[10]-23=-3S=4n-6K=2n+3Kp=C4C[M6]K*=C4+D6A tetra-capped octahedron.VE0=2[6]+2=14VEn=VE0+12n=14+12[10]=134VF=6[9]+3[8]+21[2]+1[11+2]+1=134
17. Ir6Ru3(CO)21(AuL)-1:K=6[4.5]+3[5]-21[1]+1[3.5-1]-1[0.5]=23n=6+3+1=10K(n)=23(10)2[10]-23=-3S=4n-6K=2n+3Kp=C4C[M6]K*=C4+D6A tetra-capped octahedron.VE0=2[6]+2=14VEn=VE0+12n=14+12[10]=134VF=6[9]+3[8]+21[2]+1[11+2]+1=134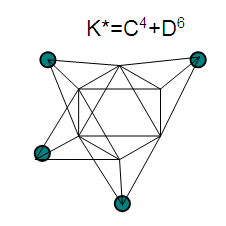
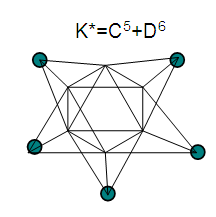
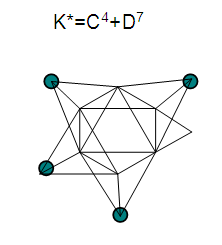
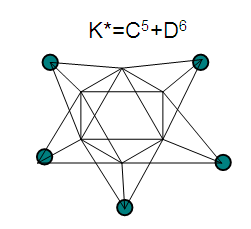
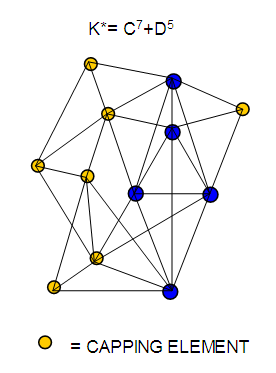
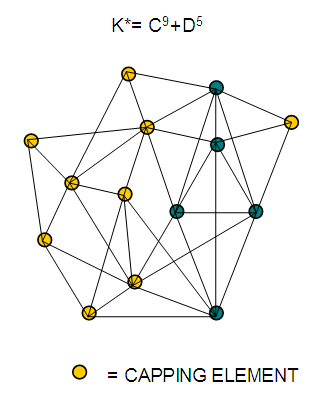
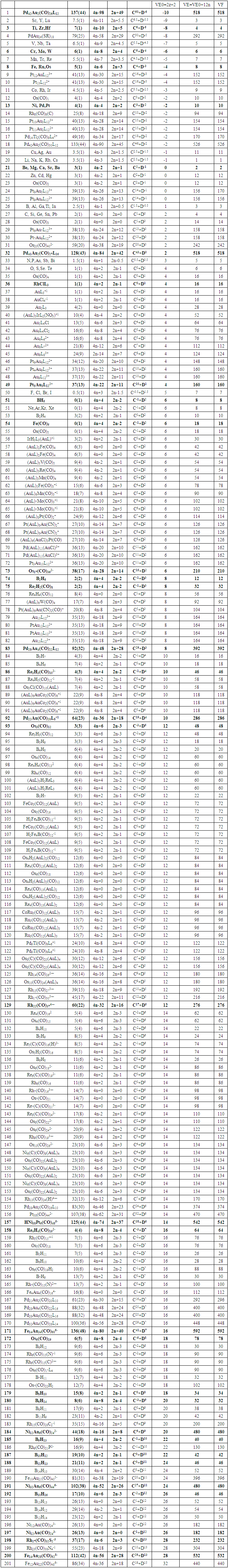
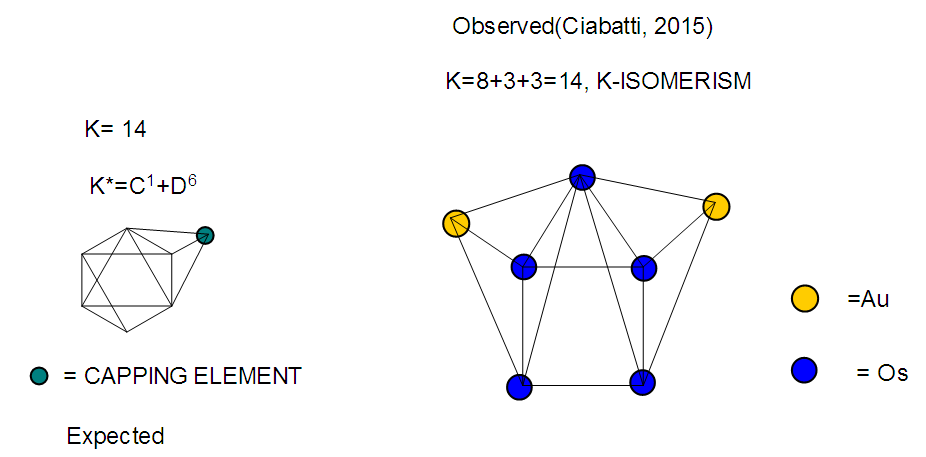
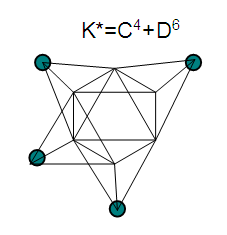
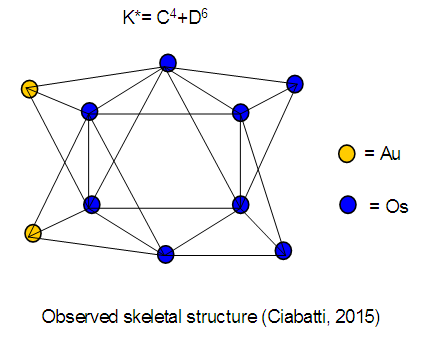
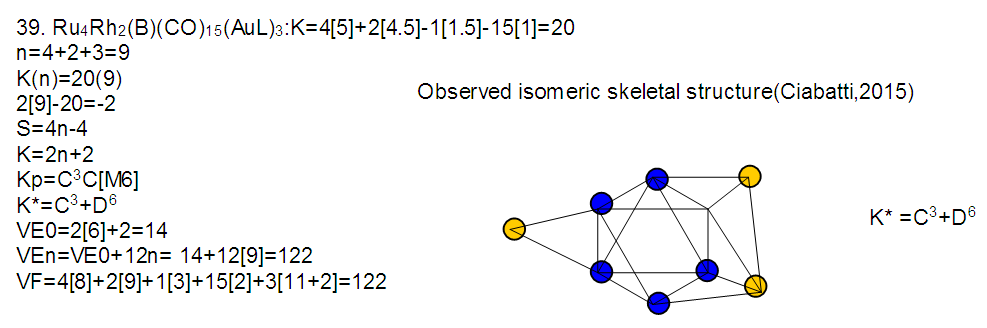
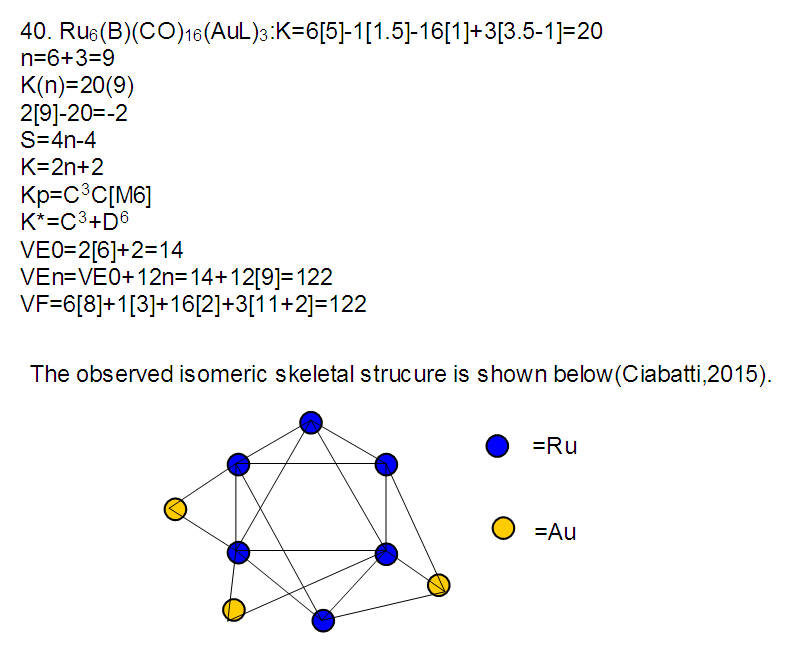
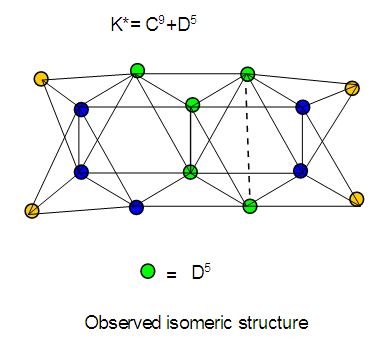 26. Ni12Au6(CO)242-: K=12[4]+6[3.5]-24-1=44,n=12+6=18,K(n)=44(18)2[18]-44=-8S=4n-16K=2n+8Kp=C9C[M9]K*=C9+D9A tri-capped trigonal prism capped nine times.K*=Cy+DzVE0=2z+2=2[9]+2=20VEn=VE0+12n=20+12[18]=236VF=12[10]+6[11]+24[2]+2=23627. Pt19(CO)24(AuL)3-1:K=19[4]-24[1]+3[3.5-1]-1[0.5]=59n=19+3=22K(n)=59(22)2[22]-59=-15S=4n-30K=2n+15Kp=C16C[M6]K*=C16+D6An octahedron capped 16 times.VE0=2[6]+2=14VEn=VE0+12n=14+12[22]=278VF=19[10]+24[2]+3[11+2]+1=27828. Fe10Au21(CO)405-:K=10[5]21[3.5]-40[1]-5[0.5]=81n=10+21=31K(n)=81(31)2[31]-81=-19S=4n-38K+2n+19Kp=C20C[M11]K*=C20+D11An octadecahedron capped 20 times.VE0=2[11]+2=24VEn=VE0+12n=24+12[31]=396VF=10[8]+21[11]+40[2]+5=39629. Pd28Au4(CO)22L16:K=28[4]+4[3.5]-22[1]-16[1]=88n=28+4=32K(n)=88(32)2[32]-88=-24S=4n-48K=2n+24Kp=C25C[M7]K*=C25+D7A pentagonal bipyramid capped 25 times.VE0=2[7]+2=16VEn=VE0+12n=16+12(32)=400VF=28[10]+4[11]+22[2]+16[2]=40030. Fe12Au22(CO)486-:K=12[5]+22[3.5]-48[1]-6[0.5]=86n=12+22=34K(n)=86(34)2[34]-86=-18S=4n-36K=2n+18Kp=C19C[M15]K*=C19+D15VE0=2z+2=2[15]+2=32VEn=VE0+12n=32+12[34]=440VF=12[8]+22[11]+48[2]+6=44031. Ni32Au6(CO)446-:K=32[4]+6[3.5]-44-3=106;N=32+6=38K(n)=106(38)2[38]-106=-26S=4n-52K=2n+26Kp=C27C[M11]K*=C27+D11An octadecahedron capped 27 times.VE0=2z+2=2[11]+2=24VEn=VE0+12n=24+12[38]=480VF=32[10]+6[11]+44[2]+6=48032. Fe14Au28(CO)528-: K=14[5]+28[3.5]-52[1]-8[0.5]=112n=14+28=42K(n)=112(42)2[42]-112=-28S=4n-56K=2n+28Kp=C29C[M13]K*=C29+D13A centered icosahedron capped 29 times.VE0=2[13]+2=28VEn=VE0+12n=28+12[42]=532VF=14[8]+28[11]+52[2]+8=53233. Fe14Au34(CO)506-:K=14[5]+34[3.5]-50[1]-6[0.5]=136n=14+34=48K(n)=136(48)2[48]-136=-40S=4n-80K=2n+40Kp=C41C[M7]K*=C41+D7A pentagonal bipyramid capped 41 times.VE0=2[7]+2=16VEn=VE0+12n=16+12[48]=592VF=14[8]+34[11]+50[2]+6=59234. Co11(C)2(CO)23-1:K=11[4.5]-2[2]-23[1]-1[0.5]=22,n=11K(n)=22(11)2[11]-22=0S=4n+0Kp=C1C[M10]K*=C1+D10A mono-capped bi-capped square anti-prism.VE0=2[z]+2=2[10]+2=22VEn=VE0+12n=22+12[11]=154VF=11[9]+2[4]+23[2]+1=154The symbol K* = C1+D10 predicts one skeletal element capping other 10 skeletal elements. This is shown in Figure 20 and is in agreement with what was reported (Ciabatti, 2015).
26. Ni12Au6(CO)242-: K=12[4]+6[3.5]-24-1=44,n=12+6=18,K(n)=44(18)2[18]-44=-8S=4n-16K=2n+8Kp=C9C[M9]K*=C9+D9A tri-capped trigonal prism capped nine times.K*=Cy+DzVE0=2z+2=2[9]+2=20VEn=VE0+12n=20+12[18]=236VF=12[10]+6[11]+24[2]+2=23627. Pt19(CO)24(AuL)3-1:K=19[4]-24[1]+3[3.5-1]-1[0.5]=59n=19+3=22K(n)=59(22)2[22]-59=-15S=4n-30K=2n+15Kp=C16C[M6]K*=C16+D6An octahedron capped 16 times.VE0=2[6]+2=14VEn=VE0+12n=14+12[22]=278VF=19[10]+24[2]+3[11+2]+1=27828. Fe10Au21(CO)405-:K=10[5]21[3.5]-40[1]-5[0.5]=81n=10+21=31K(n)=81(31)2[31]-81=-19S=4n-38K+2n+19Kp=C20C[M11]K*=C20+D11An octadecahedron capped 20 times.VE0=2[11]+2=24VEn=VE0+12n=24+12[31]=396VF=10[8]+21[11]+40[2]+5=39629. Pd28Au4(CO)22L16:K=28[4]+4[3.5]-22[1]-16[1]=88n=28+4=32K(n)=88(32)2[32]-88=-24S=4n-48K=2n+24Kp=C25C[M7]K*=C25+D7A pentagonal bipyramid capped 25 times.VE0=2[7]+2=16VEn=VE0+12n=16+12(32)=400VF=28[10]+4[11]+22[2]+16[2]=40030. Fe12Au22(CO)486-:K=12[5]+22[3.5]-48[1]-6[0.5]=86n=12+22=34K(n)=86(34)2[34]-86=-18S=4n-36K=2n+18Kp=C19C[M15]K*=C19+D15VE0=2z+2=2[15]+2=32VEn=VE0+12n=32+12[34]=440VF=12[8]+22[11]+48[2]+6=44031. Ni32Au6(CO)446-:K=32[4]+6[3.5]-44-3=106;N=32+6=38K(n)=106(38)2[38]-106=-26S=4n-52K=2n+26Kp=C27C[M11]K*=C27+D11An octadecahedron capped 27 times.VE0=2z+2=2[11]+2=24VEn=VE0+12n=24+12[38]=480VF=32[10]+6[11]+44[2]+6=48032. Fe14Au28(CO)528-: K=14[5]+28[3.5]-52[1]-8[0.5]=112n=14+28=42K(n)=112(42)2[42]-112=-28S=4n-56K=2n+28Kp=C29C[M13]K*=C29+D13A centered icosahedron capped 29 times.VE0=2[13]+2=28VEn=VE0+12n=28+12[42]=532VF=14[8]+28[11]+52[2]+8=53233. Fe14Au34(CO)506-:K=14[5]+34[3.5]-50[1]-6[0.5]=136n=14+34=48K(n)=136(48)2[48]-136=-40S=4n-80K=2n+40Kp=C41C[M7]K*=C41+D7A pentagonal bipyramid capped 41 times.VE0=2[7]+2=16VEn=VE0+12n=16+12[48]=592VF=14[8]+34[11]+50[2]+6=59234. Co11(C)2(CO)23-1:K=11[4.5]-2[2]-23[1]-1[0.5]=22,n=11K(n)=22(11)2[11]-22=0S=4n+0Kp=C1C[M10]K*=C1+D10A mono-capped bi-capped square anti-prism.VE0=2[z]+2=2[10]+2=22VEn=VE0+12n=22+12[11]=154VF=11[9]+2[4]+23[2]+1=154The symbol K* = C1+D10 predicts one skeletal element capping other 10 skeletal elements. This is shown in Figure 20 and is in agreement with what was reported (Ciabatti, 2015).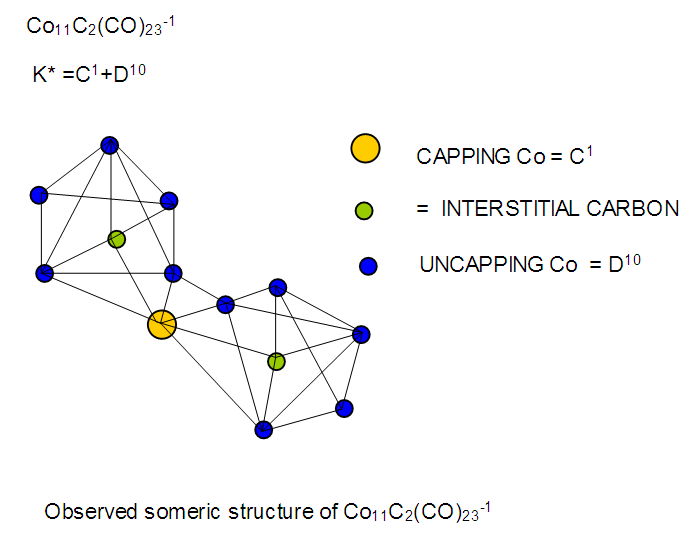
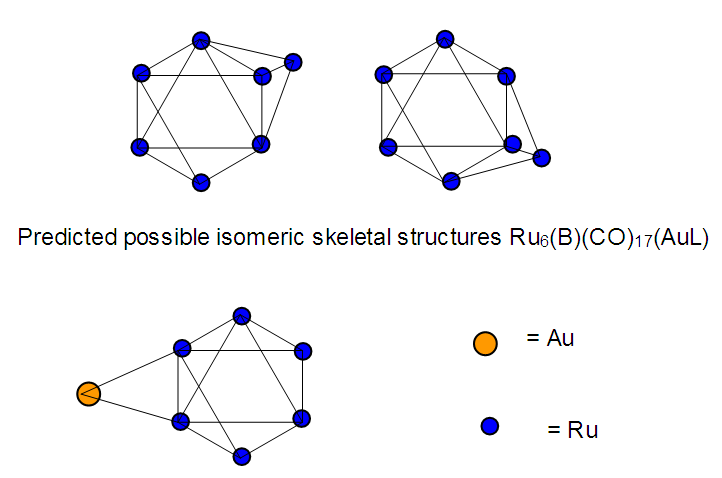
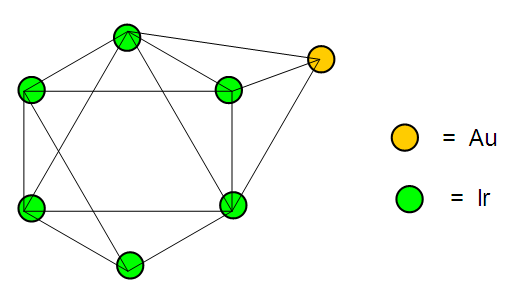
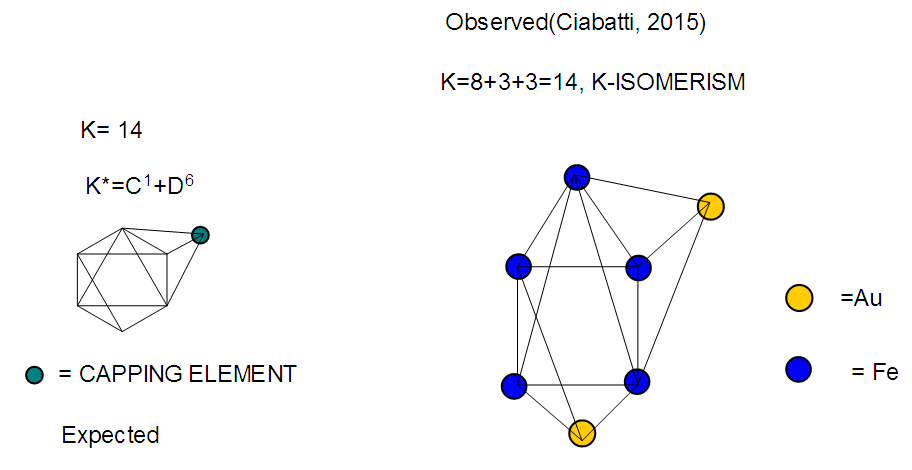
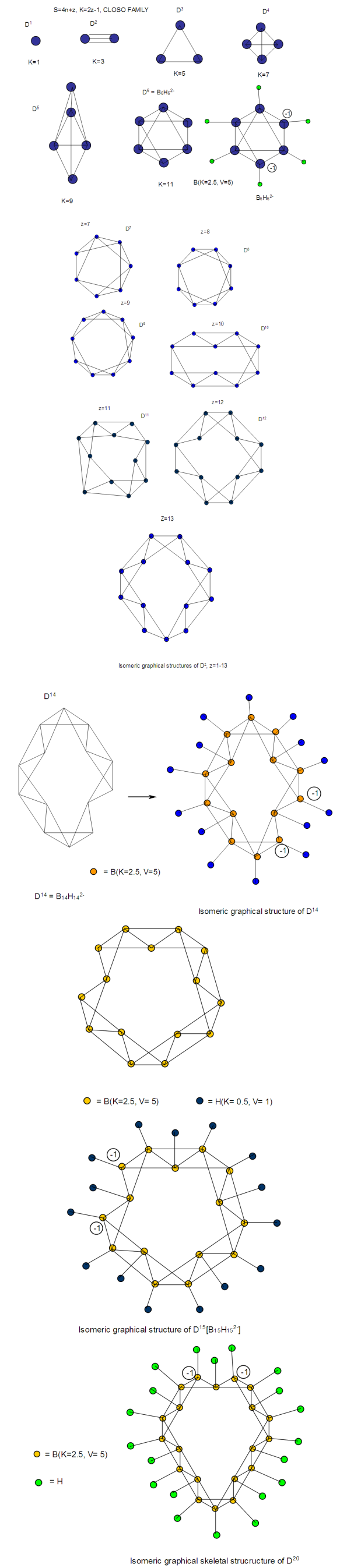
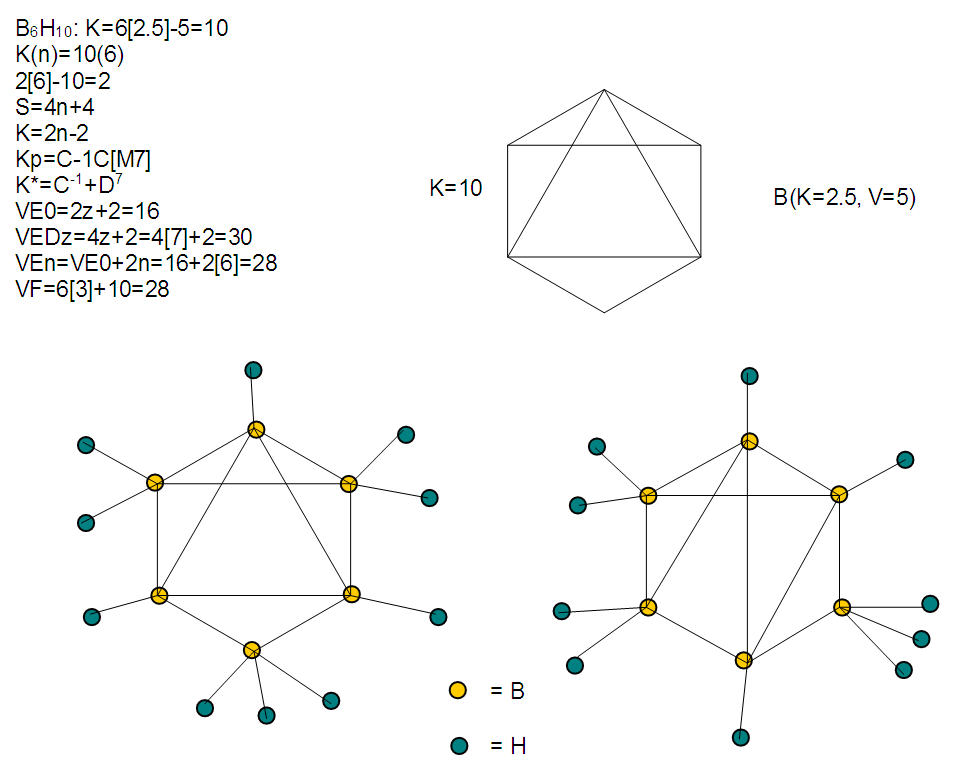
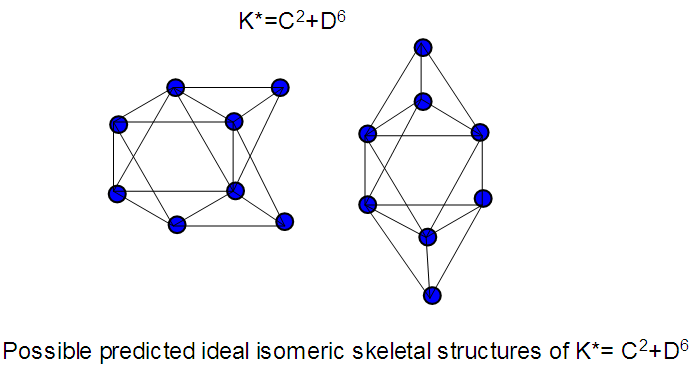 The observed skeletal structure is given in Figure 23.
The observed skeletal structure is given in Figure 23.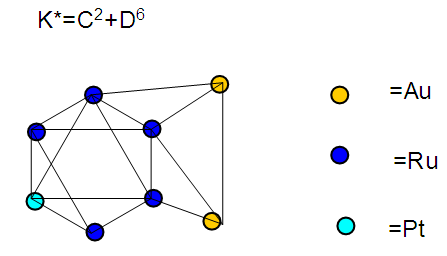
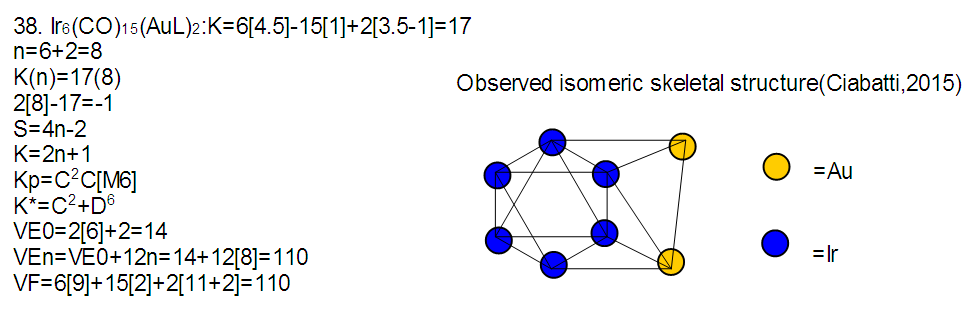
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML