-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2019; 9(2): 27-32
doi:10.5923/j.chemistry.20190902.01

Optimization Studies for Catalytic Conversion of Waste Vegetable Oil to Biodiesel
Zakariyya Uba Zango1, Haliru Aivada Kadir2, Saifullahi Shehu Imam2, 3, Atika Ibrahim Muhammad3, Ibrahim Gambo Abu1, 4
1Fundamental and Applied Sciences Department, Universiti Teknologi PETRONAS, Seri Iskandar, Perak, Malaysia
2School of Chemical Sciences, Universiti Sains Malaysia, Pulau Penang, Malaysia
3Department of Pure and Industrial Chemistry, Bayero University, Kano, Nigeria
4Department of Chemistry, Al-Qalam University Katsina, Katsina, Nigeria
Correspondence to: Haliru Aivada Kadir, School of Chemical Sciences, Universiti Sains Malaysia, Pulau Penang, Malaysia.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this study, the production of biodiesel from waste vegetable oil using a heterogeneous catalyst (CaO and KOH) was studied. In order to evaluate the catalytic efficiency, factors investigated are oil-to-methanol ratio, catalyst loading, and temperature. The density obtained for both catalysts were found to be 1.05 g/ml (CaO) and 1.07 g/ml (KOH) respectively. Viscosity values of 3.02 mm2/s and 4.31 mm2/s while acid values of 0.46 mg/g and 0.84 mg/g were obtained for CaO and KOH respectively. The highest biodiesel yield from both CaO (70%) and KOH (90%) were achieved by employing 1.5 wt% and 1.0 wt% respectively, 1:12 and 1:9 oil to methanol ratio, 60 – 65°C reaction temperature, and 4 hours reaction time. The properties of the biodiesel obtain were in conformity with ASTM limits. In order to curb the high effect of acidity, pre-treatment method such as esterification should be employed. Hence, trans-esterification process is recommended for the production of biodiesel.
Keywords: Biodiesel, Catalyst, Transesterification, Waste Vegetable Oil
Cite this paper: Zakariyya Uba Zango, Haliru Aivada Kadir, Saifullahi Shehu Imam, Atika Ibrahim Muhammad, Ibrahim Gambo Abu, Optimization Studies for Catalytic Conversion of Waste Vegetable Oil to Biodiesel, American Journal of Chemistry, Vol. 9 No. 2, 2019, pp. 27-32. doi: 10.5923/j.chemistry.20190902.01.
Article Outline
1. Introduction
- The increase in consumption of energy is as a result of industrialization and significant growth in terms of population. This is supported by the report of the U.S Energy Information Administration, which revealed that total energy consumption is significantly increasing where there exist economic growth and expanding population [1]. This led to depletion of fossil fuel, scarcity of fuel reserves, and hike in its price of non-renewable energy sources such as petrol [2]. Environmental issues such as global warming, air population, acid precipitation, alongside shortage and increase in prices of non-renewable resources led to the findings of new alternative and renewable energy sources [3]. Nowadays, biodiesel synthesis has received considerable attention from renewable sources such as non-edible and edible plant-based oils, and animal fats. It is an attractive biofuel due to its attribute such as biodegradability [4], higher flash point [9], and miscibility with petrodiesel [10], non-toxicity [12], a volatile organic compound and inherent lubricity [13, 14]. Transportation and the basic industrial sector are the major demanding bodies of energy. The transport sector is a major consumer of petroleum fuels such as diesel, gasoline, liquefied petroleum gas (LPG) and compressed natural gas (CNG) [11]. Considering the large consumptions of fossil fuels and the consequent decline on its occurrences, more demand for energy have resulted for the search of more alternative sources of harnessing the energy from other sources which are economically viable and environmentally friendly. The environmental and economic benefit of biodiesel from edible and non-edible vegetable oil puts it upfront as a future realistic fuel [15]. Reports reveal that biodiesel produced from vegetable oil sources possess similar properties (viscosity, flash point, acid value, iodine value, cetane number, etc.). These parameters reported for Biodiesels are mostly in close range proximity to that of petroleum diesel [16]. The major routes of converting Vegetable oil and animal fats to biodiesel fuel for engine consumptions involves four major processes which are direct use or blending of oils, pyrolysis, microemulsion, and transesterification techniques. Amongst these techniques, transesterification is one of the promising and widely applicable methods. This method enables the production of biodiesel fuel of high quality and resemblance to the conventional petroleum diesel. Transesterification allows agricultural and food waste to be converted to corresponding biodiesel oil which contained alkyl esters with of high viscosity [1, 5]. Biodiesel is from waste vegetable oil contained fatty acid methyl ester (FAME) which is produced from transesterification process of triglyceride with short-chain alcohols (methanol & ethanol) in the presence of a suitable catalyst which can either be an acid or base form [17, 18]. The catalysts employed during the synthesis of biodiesel could either be homogeneous or heterogeneous. However, the latter possessed certain advantages as it is mostly less toxicity, non-corrosive and environmentally friendly. Another advantage is that it is easily separated from liquid products as such it gives higher activity, and longer catalyst life [17]. According to previous findings, production of biodiesel from vegetable oils using homogenous catalyst is associated with the soapy formation and it has relatively low yield [6, 7, 8]. Recently, a heterogeneous catalyst such as alkaline earth metal oxides, alkali metal compounds on nanomaterial supports has been utilized for transesterification of vegetable oil to biodiesel [19, 20]. Literature studies have shown that CaO has been utilized as a solid base catalyst and it displayed greater attributes in terms of higher activity, mild reaction conditions as well as the catalyst cost [21]. According to one research, nano-crystalline calcium oxides were reportedly used at room temperature, and it took about 6 – 24 hours to obtain high conversion with the most active catalyst. Also, deactivation after eight cycles with soybean oil was reported [22]. Transesterification is a chemical process whereby an ester is reacted with an alcohol in the presence of a catalyst to produce a new ester and alcohol [23, 24]. Figure 1 shows the representation of a transesterification process, where, R1, R2, R3 are long fatty acid chains. As depicted above, the catalyst used can be a homogeneous or heterogeneous catalyst.
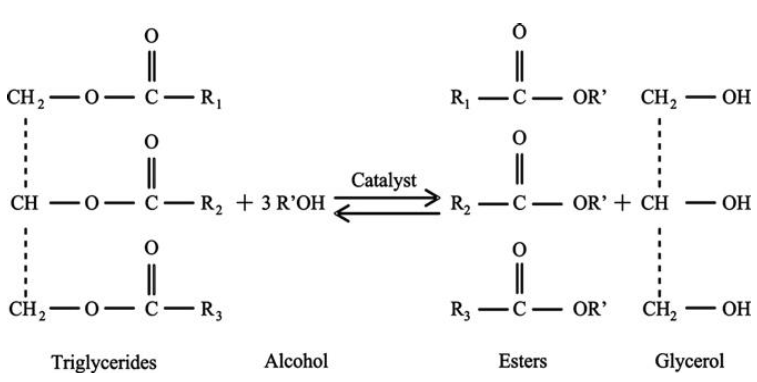 | Figure 1. General Trans-esterification Reaction |
2. Experimental Section
2.1. Materials
- The waste vegetable oil (WVO) were obtained from commercial sources (Katsina city restaurants). Vacuum filtration was employed to remove dirt content and then, heating (65°C) was done to remove the traces of water present in the WVO. Potassium hydroxide, phenolphthalein, and hydrochloric acid were purchased from Sigma Aldrich, China. Other chemical includes Methanol and ethanol were purchased from Guangzhou-Jinhuada, China, while sodium thiosulphate, calcium oxide, magnesium oxide, sodium oxide, wijs solution were all purchased from Merck Chemicals, India. The chemicals utilized were of analytical grades and used without further purification. Distilled water was utilized for preparations of aqueous solutions.
2.2. Reaction Procedure
- To carry out the conversion of waste vegetable oil to biodiesel using CaO and KOH as a catalyst, three factors such as the effect of temperature, oil to methanol ratio, and catalyst loading were investigated. Transesterification reaction was carried out in a well-calibrated water bath with the set temperature. The reaction procedure was as follows: First, the catalyst was dispersed into a test tube containing methanol and stirred. Then 2 g of WVO was added into the mixture and covered tightly to avoid spillage, the tubes were lowered into a well-calibrated water bath and the temperatures were set accordingly. Temperature range of 55 – 65°C, oil to methanol ratio of 1:6 - 1:12 and catalyst loading of the range 0.5 – 1.5 wt% were employed. Reaction time stood at 4 hours and 40 experimental runs were conducted (20 each for both catalysts). Upon completion, the sample was collected and allowed to cool down for 15 – 20 mins, centrifuged and a two-phase system was observed which was carefully separated via decantation. The bottom layer consists of glycerine and catalyst, while the top layer was made up of a mixture of biodiesel and little amount of unreacted methanol. During the course of this work, experiments were conducted by the following identical procedure but based on different experimental or operating conditions.
2.3. Physiochemical Analysis
- The physiochemical analysis carried out includes percentage yield, determination of density, iodine value, acid value, and viscosity. These factors were utilized to ascertain the proximity (how close) in terms of comparison with the properties of conventional biodiesel. The percentage yield of oil was deduced by weighing the mass of biodiesel produced, hence represented by the following equation:
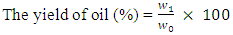 | (1) |
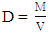 | (2) |
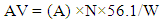 | (3) |
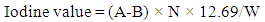 | (4) |
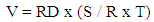 | (5) |
3. Results and Discussion
3.1. Effect of Reaction Conditions
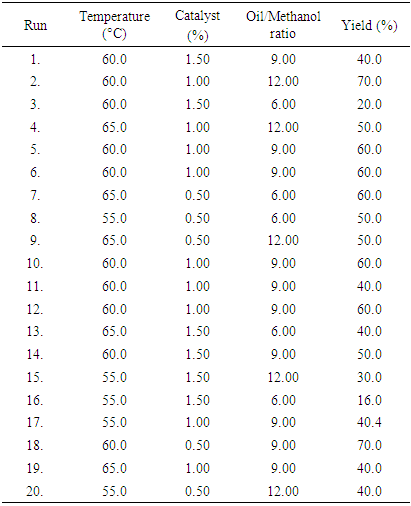
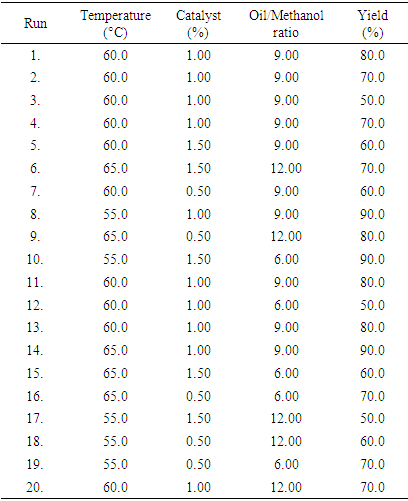
- The response for Biodiesel produced by using CaO and KOH as a catalyst is obtained using the Box-Behnken Design expert software, which is presented in Table 1 and 2 respectively. It shows how the reaction conditions (oil/methanol ratio, temperature, and catalyst loading) via response surface method influence the % yield of biodiesel. Forty (40) separate experimental runs were conducted, 20 runs for each individual catalyst. It was observed that the percent yield of biodiesel obtained for both catalysts is quite promising.
|
|
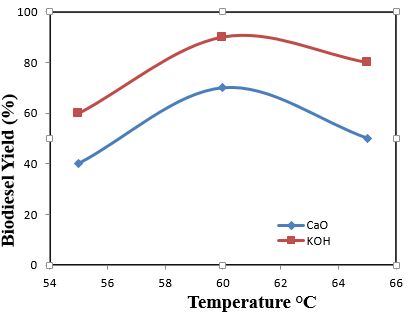 | Figure 2. Plot indicating the relation between Temperature and Yield |
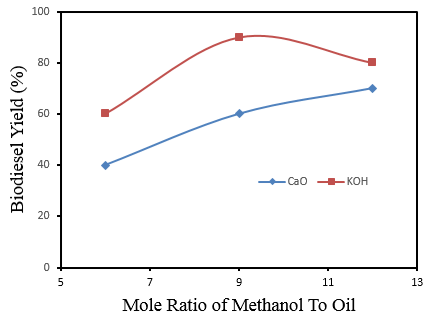 | Figure 3. The plot of the Molar ratio of Methanol/oil Vs Yield |
3.2. Outcomes of Physicochemical Analysis
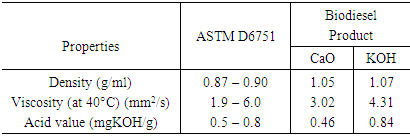
- The properties investigate are density, iodine value, acid value, and viscosity. The values obtained for these parameters were compared to the American Society for Testing and Materials (ASTM) specifications. The density value obtained from the use of CaO and KOH catalyst was found to be 1.05 g/ml and 1.07 g/ml respectively. These values are in conformity with the ASTM standards for density which is around 0.9 g/ml. Iodine value simply means the degree of the unsaturated bond of fats and oils or biodiesel fuel. The iodine values of 7.29 and 8.53 were obtained from the use of CaO and KOH as catalyst respectively. The KOH catalyst has a higher iodine value compared to CaO. It has been reported that one of the technical problem associated with biodiesel is its susceptibility to oxidation [28]. Considering the values obtained, it does indicate that, the tendency of oxidation occurring is low. Viscosity is a vital parameter that has an effect on the performance of the fuel in engines. It is a priority to obtain biodiesel with fewer viscosity values since one of the major reason why transesterification of vegetable oil or fat is carried out is due to the operational problems such as engine deposits resulting from high viscosity values. The viscosity values obtained were 3.02 mm2/s and 4.31 mm2/s at 40°C for CaO and KOH respectively. These two values are within the stipulated ASTM standard for biodiesel. Hence, connotes the possibility of good conversion efficiency.
|
4. Conclusions
- In summary, the aim of the study is to evaluate the efficiency of CaO and KOH as a catalyst in the synthesis of biodiesel from waste vegetable oil (WVO). Factors utilized were temperature, oil/methanol ratio, and catalyst loading while the biodiesel properties analyzed were density, viscosity, acid value, iodine value, and yield. The results obtained were quite promising and reveals the potential of both catalysts. The properties of the biodiesel obtained were found to tally with the ASTM specified standards. Higher yield was obtained for biodiesel resulting KOH catalyst, as well as higher values for properties analyzed. Acid values obtained were 0.46 mgKOH/g and 0.84 mgKOH/g for CaO and KOH respectively. The higher acid value observed for KOH might be due to variation in operational conditions. The conversion efficiency of the biodiesel was revealed by the viscosity value which was found to be 3.02mm2/s and 4.31mm2/s at 40°C for CaO and KOH respectively. These two values are within the stipulated ASTM standard for biodiesel and it relates the good tendency of both catalysts. Hence, in entirety, both catalysts displayed relevant applicability and the biodiesel obtained from waste vegetable oil displayed properties whose values conform to ASTM specified standards.
ACKNOWLEDGEMENTS
- The authors wish to acknowledge the efforts of the Department of Chemistry, Al-Qalam University Katsina for purchasing the reagents and apparatus used and providing all the necessary support during the course of this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML