-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2019; 9(1): 21-25
doi:10.5923/j.chemistry.20190901.03

Synthesis and Structure Determination of Some Schiff Base Metal Complexes with Investigating Antibacterial Activity
Arjun Bhowmick1, Merazul Islam1, Rina Bhowmick2, Mamon Sarkar2, Abu Shibly3, Emdad Hossain4
1Department of Chemistry, Mawlana Bhashani Science and Technology University, Santosh, Tangail, Bangladesh
2Department of Chemistry, University of South Dakota, Vermillion, South Dakota, USA
3Department of Biotechnology and Genetic Engineering, Mawlana Bhashani Science and Technology University, Santosh, Tangail, Bangladesh
4Wazed Mia Science Research Center, Jahangirnagar University, Savar, Dhaka, Bangladesh
Correspondence to: Arjun Bhowmick, Department of Chemistry, Mawlana Bhashani Science and Technology University, Santosh, Tangail, Bangladesh.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Highly reactive four dentate Schiff base ligand L-1 has been prepared and used to synthesize a series of metal [Mg(II), Cu(II), Ni(II), Zn(II)] coordinating complexes 1-4. Spectroscopic and analytical techniques were used to elucidate the structures of all compounds. To investigate antibacterial activities of the complexes, E. Coli, Shigella Dysenteriae and Shigella Sonnei gram negative bacteria are used while streptomycin and ciprofloxacin antibiotics were used as standards. All complexes have shown satisfactory results as antibacterial agents to inhibit bacterial growth.
Keywords: Antibiotic, Conductance, Complexes, Bacteria, Ligand
Cite this paper: Arjun Bhowmick, Merazul Islam, Rina Bhowmick, Mamon Sarkar, Abu Shibly, Emdad Hossain, Synthesis and Structure Determination of Some Schiff Base Metal Complexes with Investigating Antibacterial Activity, American Journal of Chemistry, Vol. 9 No. 1, 2019, pp. 21-25. doi: 10.5923/j.chemistry.20190901.03.
Article Outline
1. Introduction
- Metal and ligand coordination complexes have both attracted considerable attention due to their various applications in everyday life [1] and biological [2] processes. Some compounds were synthesized due to their interesting reactivity with different substrates [3] and are also to reveal their chemistry [4]. In addition, metal complexes show good catalytic activity [5] and pharmacological properties [6]. Synthesis of these complexes involved using mono, bis, tris, and multi dentate ligands. For example, the ability of thiosemicarbazones to form stable complexes with transition metal ions make them versatile pharmacophores [7]. Additionally, Schiff bases are another class of organic ligands widely studied due to their ease of synthesis, structural diversities, and usable characteristics [8]. Schiff bases are compounds possessing imine bonds (C=N) formed when an aldehyde or ketone carbonyl (C=O) reacts with a primary amine (R-NH2). These compounds are used as pigments, catalyst, organic intermediate and polymer stabilizer [9].In Schiff base complexes, phenolic hydroxyl moieties easily give up protons to coordinate with metals and sometimes dimers form by the M…O axial coordination process [10]. The flexibility of ethylenediamine backbones is well studied [11] and, some diamino aromatic Schiff base complexes are being studied for possible tuning properties via introduction of electron withdrawing and donating substituents on the aromatic ring [12].Nowadays, the development of metal complexes and their use to solve worldwide problems such as spreading infectious diseases and establishing resistance against pathogenic attack [13] is attracting researchers. Hence, these compounds are used in various forms like soaps, detergents, household cleaners, paints, kitchenware, and school and hospital utensils [14]. In addition, a wide range of activities such as rodenticide, anti tubercular, herbicidal, insecticidal and anthelmintic are displayed by ligands and their metal coordination complexes [15]. This research is concentrating on Schiff base ligands and metal complexes, because it is already known that Schiff base metal complexes have potential biological activities such antitumor, anticancer [16], anticonvulsant, anti-inflammatory anti-tuberculous, analgesic, and antidepressant activities. But due to laboratory limitation, we have focused this research only on antibacterial activity. The ligands L-1 is a tetradentate ligand, but some dimeric complexes were also formed. One promising feature of these Schiff bases is that phenolic hydroxyl groups and imino nitrogens form strong coordination bonds resulting in structural stability and bridging activity.
2. Experimental
2.1. Material and Methodology
- All the chemicals were used of analytical grade and few solvents were purified by following standard procedures. The molar conductance at 10-3 molar dilution was measured by Elico-Conductometer. The IR spectra were recorded on Perkin-Elmer FTIR spectrophotometer in KBr pellets. The UV-visible spectra were recorded in CDCl3 and CH3OH solvent on Beckman DU-64 spectrophotometer with quartz cells of 1 cm path length. 1H NMR spectra were recorded in CDCl3 and CD3OD on a Bruker Advance 400 MHz instrument. Melting point was determined by Fisher melting point apparatus (Dimension 35×20×45, up to 350°C).
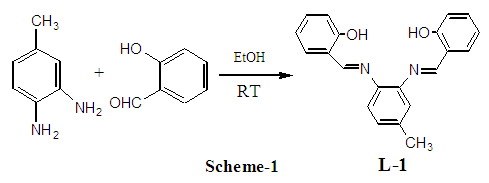
2.2. Synthesis of the Ligand 3,4-bis(salicylidene diimine)toluene[C21H18N2O2] (L-1)
- It was stirred acidic (0.5 mL glacial acetic acid) ethanolic (30 mL) salicylaldehyde (10.2 g, 83.52 mmol) for 10 min in a 250 mL round bottom flask and to this solution ethanolic (20 mL) 3,4-toluenediamine (5.0 g, 40.93 mmol) was added drop wise and finally, yellow color 3,4-bis (salicylidene diimine)toluene powders were collected by filtering (scheme 1). The resulting crude product was recrystallized in CHCl3 with (6.45 g, 47.99%) yield at room temperature. Spectral data: Anal. Cal. For C21H18N2O2: C, 76.81; H, 4.91; N, 9.74. Found: C, 77.98; H, 5.18; N, 8.10. IR (υ CO, KBr): 3440 (OH, b), 3060 (ArC-H, w), 3017 (N=C-H, w), 2983 (CH3, w), 2856 (CH3, w), 1617 (C=N, vs), 1570 (ArC=C,s), 1348 (C-N, b), 1148 (C-O, vs). 1H NMR (CDCl3): δ 13.15 (s, 2H), δ 13.1 (s, 1H), δ 8.66 (s, 1H), δ 8.65 (s, 1H) δ 7.4-6.9 (m, 11H), 2.4 (s, 3H). Melting Point: 122°C.
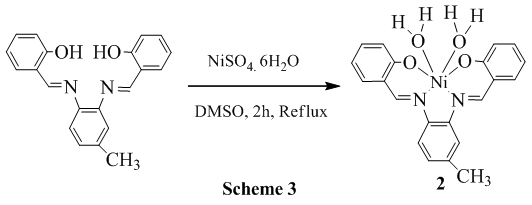
2.3. Reaction of 3,4-bis(salicylidene diimine)toluene with NiSO4. 6H2O
- 0.3958 g (1.51 mmol) of NiSO4.6H2O was dissolved in 20 mL DMSO and stirred for 30 min and in another pot 0.500 g (1.52 mmol) of 3,4-bis(Salicylidene diimine)toluene was also dissolved in 10 mL DMSO and it was added to the metal salt solution and the resulting solution was refluxed for 2 h and finally, radish brown crystal was separated (scheme 3) and recrystallized in CHCl3 (0.4861 g, 76.27%). Spectral data: Anal. Cal. For C21H20N2O4Ni: C, 59.62; H, 4.77; N, 6.62; Found: C, 60.32; H, 4.98; N, 5.93; IR (υ CO, KBr): 3417 (H2O-OH, b), 3044 (ArC-H, w), 3010 (N=CH, w), 2922 (CH3, w), 2853 (CH3, w), 1609 (C=N, vs), 1582 (ArC=C, s), 1332 (C-O, m), 1352 (C-N, vs), 580 (Ni-N, s), 460 (Ni-O, m). 1H NMR (CDCl3): δ 11.04 (s, 1H), 9.93 (s, 1H), δ 8.2-6.7 (m, 11H), δ 2.6 (s, 3H), δ 1.6 (s, 4H). Melting Pont: > 300°C. Molecular conductance (μs/cm): 20.1 (DMSO), 0 (CHCl3). UV-Visible (λmax, CHCl3): 260, 379, 478.
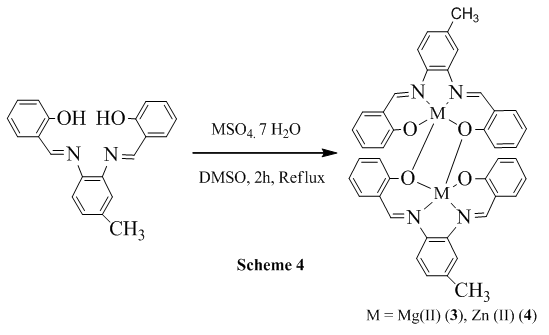
2.4. Reaction of 3,4-bis(salicylidene diimine)toluene ZnSO4.7H2O
- 0.4330 g (1.51 mmol) of ZnSO4.6H2O was dissolved in 20 mL DMSO and stirred for 30 min and in another pot 0.500 g (1.51 mmol) of 3,4-bis(Salicylidene diimine)toluene was also dissolved in 10 mL DMSO and it was added to the metal salt solution and the resulting solution was refluxed for 2 h and finally, white-yellow color crystal was separated (scheme 4) and recrystallized in CHCl3 (0.3443 g, 28.93%). Spectral data: Anal. Cal. For C42H32N4O4Zn2: C, 64.06; H, 4.10; N, 7.11. Found: C, 64.86; H, 5.01; N, 6.93. IR (υ CO, KBr): 3056 (ArC-H, w), 3002 (N=CH, w), 2918 (CH3, w), 2853 (CH3, w), 1621 (C=N, vs), 1532 (ArC=C, s), 1317 (C-O, m), 1180 (C-N, vs), 534 (Zn-N, m), 465 (Zn-O, m). 1H NMR (CDCl3): δ 8.7 (1H, s), 8.6 (1H, s), δ 7.5-6.7 (11H, m), δ 2.4 (3H, s). Melting Pont: > 300°C. Molecular conductance (μs/cm): 6.4 (DMSO), 0 (CHCl3). UV-Visible (λmax, CHCl3): 295, 389.
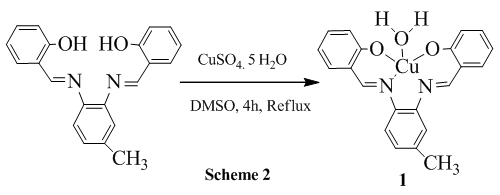
2.5. Reaction of 3,4-bis(Salicylidene diimine)toluene with CuSO4.5H2O
- 0.3760 g (1.52 mmol) of CuSO4.5H2O was dissolved in 20 mL DMSO and stirred for 30 min and in another pot 0.500 g (1.52 mmol) of 3,4-bis(Salicylidene diimine)toluene was also dissolved in 10 mL DMSO and it was added to the metal salt solution and the resulting solution was refluxed for 2 h and finally, blackish brown crystal was separated (scheme 2) and recrystallized in CHCl3 (0.4341g, 70.24%). Spectral data: Anal. Cal. For C21H18N2O3Cu: C, 61.54; H, 4.43; N, 6.83. Found: C, 61.99; H, 5.30; N, 5.79. IR (υ CO, KBr): 3421 (H2O-OH, bw), 3056 (ArC-H, w), 3010 (N=CH, w), 2964 (CH3, w), 2863 (CH3, w), 1609 (C=N, vs), 1590 (ArC=C, s), 1333 (C-O, m), 1140 (C-N, vs), 510 (Cu-N, m), 450 (Cu-O, m). 1H NMR (CDCl3): δ 11.04 (s, 1H), 9.9 (s, 1H), δ 7.8-7.0 (m, 11H), δ 2.6 (s, 3H), 1.6 (s, 2H). Melting Pont: > 300°C. Molecular conductance (μs/cm): 6.3 (DMSO), 0 (CHCl3). UV-Visible (λmax, CHCl3): 350, 424.
2.6. Reaction of 3,4-bis(Salicylidene diimine)toluene with MgSO4.7H2O
- 0.3712 g (1.51 mmol) of MgSO4.7H2O was dissolved in 20 mL DMSO and stirred for 30 min and in another pot 0.500 g (1.51 mmol) of 3,4-bis(Salicylidene diimine)toluene was also dissolved in 10 mL DMSO and it was added to the metal salt solution and the resulting solution was refluxed for 2 h and finally, white color crystal was separated (scheme 4) and recrystallized in CHCl3 (0.1242 g, 11.72%). Spectral data: Anal. Cal. For C42H32N4O4Mg2: C, 71.52; H, 4.57; N, 3.97. Found: C, 72.20; H, 5.50; N, 4.80. IR (υ CO, KBr): 3075 (ArC-H, w), 3011 (N=CH, vw), 2940 (CH3, w), 2853 (CH3, w), 1636 (C=N, vs), 1544 (ArC=C, s), 1260 (C-O, m), 1229 (C-N, vs), 510 (Mg-N, m), 430 (Mg-O, m). 1H NMR (CDCl3): δ 8.4 (1H, s), δ 8.2 (1H, s), δ 7.6-6.9 (11H, m), δ 2.6 (3H, s). Melting Point: 260°C. Molecular conductance (μs/cm): 10.2 (DMSO), 0 (CHCl3). UV-Visible (λmax, CHCl3): 375, 380.
2.7. Sample Preparation Procedure for Antibacterial Study
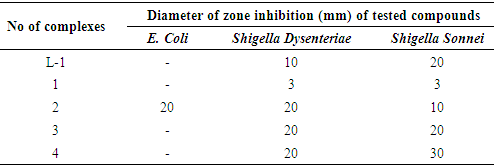
- Discs of about 4 mm in diameter were cut by a punching machine from Whatman No.1 filter paper. The discs were placed in a Petri dish and sterilized by autoclave, dried in an oven at 180°C. 1 mg of solid sample was dissolved in 1 mL volume of CHCl3 to give a solution of known concentration (1 mg/mL). CHCl3 was chosen as the solvent because, in addition to that it can be used in dissolving the solid sample completely while Zn complexes were dissolved in MeOH, both CHCl3 and MeOH have inhibitory effects on the cultures. Streptomycin and ciprofloxacin antibiotic discs with concentration of 50 μg/disc were used as standards in the study. To prepare the required volume of this medium, calculated amounts of each constituent was taken in a conical flask and distilled water was added to make the required volume. The contents were heated in a water bath to make a clear solution. The pH (at 25°C) was adjusted to 7.2-7.6 using NaOH and HCl (0.1 M). 10 mL and 5 mL of the medium was then transferred in to screw cap test tubes to prepare plates and slants, respectively. The test tubes were then capped and sterilized by autoclaving at 15-lbs. pressure/sq. inch at 121°C for 20 minutes. The slants were used for making fresh cultures for bacteria and fungi that were in turn used for sensitivity studies. Test tube slants of NA (nutrient agar) medium was prepared for culture maintenance. Then small amounts of the collected microorganisms were transferred to the test tubes with the help of sterilized needles. Several test tubes were freshly cleaned for each bacterial pathogen. The slants were inoculated at room temperature under standard laboratory conditions. In order to avoid any type of contamination and cross contamination by the test organisms, the antimicrobial screening was done in Laminar Hood and all necessary precautions were maintained. UV light was switched on one hour before working in the Laminar Hood. Petri dishes and other glassware’s were sterilized by autoclaving at a temperature of 121°C and a pressure of 15-lbs./sq. inch for 20 minutes. The sample discs (socking with 0.1 mL test sample solution), the standard antibiotic discs, and the control discs were placed gently on the previously marked zones in the agar plates pre-inoculated with test bacteria. The plates were then kept in a refrigerator at 4°C for about 24 hours upside down to allow enough diffusion of the materials from the discs to the surrounding agar medium. The plates were then inverted and kept in an incubator at 37°C for 24 h. After incubation, the diameter of the zone of inhibition were observed and measured in mm by a transparent scale. Results obtained from these were listed in Table 1.
3. Result and Discussion
- The four dentate ligand L-1 was synthesized following standard procedure as shown in scheme-1 and the impure yellow powders were purified through recrystallization in CHCl3. Metal complexes were synthesized using this ligand and all compounds were characterized by IR, 1H NMR, elemental analysis, melting point, UV-Visible spectra, and conductivity measurements. The formation of the imine bond was confirmed from carbon-hydrogen bond stretching (N=CH) at 3017 cm-1 and carbon nitrogen at 1636 cm-1 (C=N) for L-1 and no peak was found for carbonyl group stretching. In addition, L-1 gave IR stretching frequencies at 2983 cm-1 and 2856 cm-1 due to asymmetric and symmetric stretching of -CH3, respectively. Since the chemical environment of the L-1 is not same, imine protons gave two δ values at 8.66 ppm and 8.65 ppm. The phenolic two protons came at δ 13.15 ppm and 13.10 ppm. The melting point was determined at 122-124°C for their final identification. The reaction of the formation of Ni (II), Cu (II), Zn (II) and Mg (II) are shown in scheme 2, scheme 3 and scheme 4. The detail reaction procedure and characterization data are shown in experimental sections 2.2-2.6. After dissolving the metal complexes in CHCl3, the unreacted metal salt was separated by filtering off. In IR, all complexes gave distinctive absorption bands and the broad peak appearing between 3444-3417 cm-1 for the coordinating water, aromatic -CH stretching (Ar-H) bands in between 3075-3044 cm-1, imine carbon-hydrogen stretching (N=CH) 3020-3002 cm-1 and 1609-1636 cm-1 (C=N), aliphatic -CH stretching values (-CH3) 2983-2913 cm-1 (asymmetric) and 2863-2827 cm-1 (symmetric). No coordinating water was found in complex 3 and complex 4, this was confirmed from the 1H NMR proton integration and elemental analysis values.In 1H NMR, it was seen that the aromatic protons appeared between δ 6.9-8.2 ppm, imine hydrogens 8.1-9.9 ppm. None of the complex showed phenolic hydroxyl peak since they were coordinated with metals. Aliphatic protons (-CH3) give peaks in between δ 2.4-2.6 ppm and coordinated water protons at 1.6 ppm. The Mg (II) and Zn (II) formed a dimeric complex. Digital melting point apparatus was used to determine melting points and found above 300°C, except in 3 (260°C). The ionic conductivity (µscm-1) was measured in CHCl3 and DMSO for all the complexes. Weak conductance has found weak conductance for all complexes and in CHCl3 they showed zero conductivity. All metal complexes gave excellent (λmax) UV-Visible absorption bands due to the presence of metal. From those absorption bands it was possible to identify the metals.
4. Antibacterial Activity
- The ligand L-1 and complexes 1-4 were tested for E. Coli, Shigella Dysenteriae, and Shigella Sonnei gram negative bacteria. It was found that complex 2 only showed activity against E. Coli bacteria (Table 1) compared to a ciprofloxacin standard. Conversely, all complexes showed good activity against Shigella Dysenteriae, Shigella Sonnei bacteria, where streptomycin was used as an antibiotic and standard.
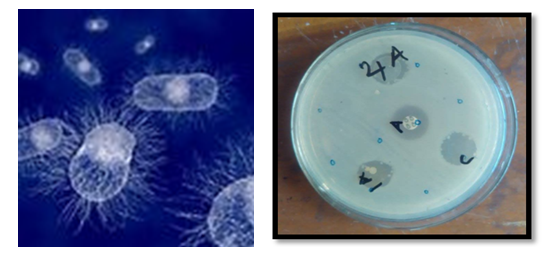 | Figure 1. Shigella Sonnei gram netative bacteria (Left), antibacterial disc showing inhibition zone (Right), 4A (Ni-complex), A (antibiotic), 1A (Cu-complex), C (CDCl3) |
|
5. Conclusions
- All complexes were characterized properly by using spectroscopic and analytical tools. Zn (II) and Mg (II) complexes formed unusual dimeric complexes. The antibacterial activity was found against Shigella Dysenteriae and Shigella Sonnei gram negative bacteria. Finally, this research provides a potential application of metal complexes as medicinal agents although further studies needed to understand the minimum lethal doses.
ACKNOWLEDGMENTS
- We are grateful to the Department of Chemistry, Mawlana Bhashani Science and Technology University, Bangladesh for supporting this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML