-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2019; 9(1): 13-20
doi:10.5923/j.chemistry.20190901.02

Comparative Study on the Cosmeceutical Properties of Oils from Dacryodes edulis (African Pear) and Persea americana (Avocado) Fruits
Iniobong S. Enengedi1, Okon D. Ekpa2, Magdalene E. Ikpi2
1Department of Chemistry, Akwa Ibom State University, Ikot Akpaden, Akwa Ibom State, Nigeria
2Department of Pure and Applied Chemistry, University of Calabar, Calabar, Cross River State, Nigeria
Correspondence to: Iniobong S. Enengedi, Department of Chemistry, Akwa Ibom State University, Ikot Akpaden, Akwa Ibom State, Nigeria.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

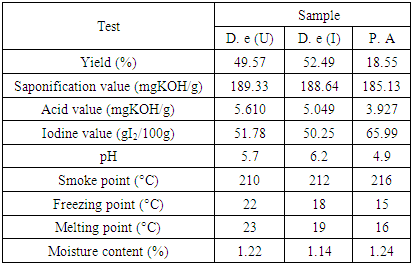
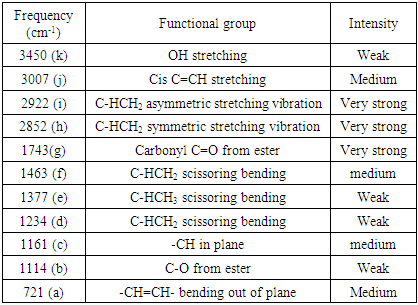
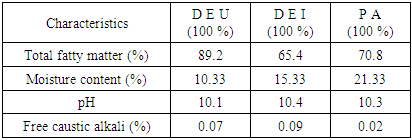
Fruits are major sources of oils for human nutrition as well as for several industrial purposes. The properties of Dacryodes edulis fruit oils from two locations (Uyo and Ikom) were compared to those of Persea americana, a widely used oil in cosmeceutical formulations. All the oils extracted from each of these fruits were liquid at room temperature. The oil yield of P. americana (18.55%) was low in comparison to those of D. edulis-Uyo (49.57%) and D. edulis-Ikom (52.49%). The saponification value of D. edulis-Uyo, D. edulis-Ikom and P. americana oils were 189.33 mgKOH/g, 188.64 mgKOH/g and 185.13 mgKOH/g respectively, indicating their potential application in soap making. Fourier Transform Infrared (FT-IR) spectra of P. americana and D. edulis oils appear very similar, however, they revealed slight differences. Soap produced with D. edulis oil from Uyo gave the best quality soap considering the high fatty matter of 89.2% as compared to that of D. edulis soap from Ikom oil (65.4%) and soap from P. americana oil (70.8%). The oils from these fruits could be used as emollients in cosmeceuticals. The higher oil yield of the fruits of D. edulis than P. americana, with similar functional groups (C-HCH3, C=O, C-O, C-HCH2 and -CH=CH), could project D. edulis oils as possible substitute for P. americana oil in cosmeceutical formulations.
Keywords: Dacryodes edulis, P. americana, Oils, Cosmeceutical formulations
Cite this paper: Iniobong S. Enengedi, Okon D. Ekpa, Magdalene E. Ikpi, Comparative Study on the Cosmeceutical Properties of Oils from Dacryodes edulis (African Pear) and Persea americana (Avocado) Fruits, American Journal of Chemistry, Vol. 9 No. 1, 2019, pp. 13-20. doi: 10.5923/j.chemistry.20190901.02.
Article Outline
1. Introduction
- Vegetable oils have been used on skin for cosmetic and medical purposes. They act as protective barriers to the skin by occlusive effect, allowing the skin to retain moisture, thereby, resulting in hydration of the skin [1]. Dacryodes edulis (African pear) is an indigenous fruit tree in the humid low lands and plateau regions of West, Central African and Gulf of Guinea countries [2]. The fruits of D. edulis can be eaten either raw, cooked in salt water, roasted in hot ash or grilled in the oven [3]. D. edulis fruit could serve the dual purpose of being a source of minerals and vitamins to human nutrition and as a raw material for industries, if properly harnessed. The pulp and seed of D. edulis had been found to contain reasonable amounts of oil [4, 5]. [4] Reported the percentage oil content in D. e. var. edulis and D. e. var. parvicarpa to be 68.29% and 54.68% and [5] reported 32.56% oil content for unspecified variety of D. edulis. [2] Reported 32.62 - 35.05% oil content of D. edulis at different stages of fruit development. [6] Reported the fatty composition of D. edulis oil to be rich in saturated fatty acids, having palmitic acid (44.31%) and stearic acid (8.07%), and unsaturated fatty acids having oleic acid (42.45%) and linoleic acid (5.17%). D. edulis oil from ripe fruits had been used as a precursor for synthesis of surface coating driers [6].Persea americana (avocado) is a medium sized tree, measuring about 9-20 meters in height. It is widely grown worldwide for fruits on large scale in various subtropical countries and are generally recognized as a popular and healthy food source supplying proteins and lipids to the human diet [7]. The fruit is not sweet but fatty, almost distinctly, yet subtly flavoured, and of smooth, almost creamy texture [8]. P. americana is a good source of oil, containing monounsaturated fat. Its oil content varies depending on its varieties and the period of extraction of the oil by cold-press processs [9]. [9] Reported the oil content of 27.12%, pH of 5.7; iodine value of 37.26 g/100g and saponification value of 219.20 mgKOH/g from the fruit pulp.The binding of water in the stratum corneum can become compromised and ineffective. In this case it is helpful to reduce the transepidermal water loss by applying occlusive films. Mineral oil should be used; however, the benefits of a natural vegetable oil is preferred [10]. Natural oils are of significant nutritional importance and are also desirable emollients for skin care applications. Natural oils are good sources for tocopherols and phytosterols, components offering both antioxidant activity and bioactivity for skin care applications [11]. D. edulis and P. americana oils could be of great importance in cosmeceuticals intended for daily care of the face and body. Deficiency in oil could result in excessive dryness of the skin. D. edulis and P. americana oils could serve as a cosmetic base which would prevent water loss through the skin, mainly by means of making a protective layer on the epidermis. They could also soften the skin, thereby reducing fine wrinkles.This is the first comparative study to project D. edulis oils as possible substitute for P. americana oil in cosmeceutical formulations.
2. Materials and Methods
2.1. Sample Collection and Identification
- D. edulis fruit was collected from Uyo (A) in Akwa Ibom State and Ikom (B) in Cross River State, Nigeria. Also P. americana fruit (C) was collected from Oron in Akwa Ibom State, Nigeria. The samples were transferred into polyethelene bags, labelled properly and taken to the laboratory for identification and preparation. The fruits were identified and authenticated by a Taxonomist in the Department of Botany and Ecological Studies, University of Uyo, Akwa Ibom State, Nigeria. Voucher specimens were deposited at the herbarium with the number, UUH 3541 and 3546 for D. edulis and P. americana respectively.
 | Figure 1. Experimental samples: D. edulis fruits from Uyo (A), D. edulis fruits from Ikom (B) and P. americana fruits (C) |
2.2. Oil Extraction
- Ripe fruits of D. edulis from Uyo and Ikom were washed with water, put in polyethylene bags for 24 hours for the fruit to soften. The pulps were removed and mashed using mortar and pestle to have a smooth paste. Also, unripe fruits of P. americana were kept for about 48 hours at room temperature until they were ripened. The fruits were cut open with a stainless-steel knife to remove the seed from the pulp, and the skin was removed from the pulp. The pulps were mashed using mortar and pestle to have a smooth paste. Each of the mashed samples were spread on stainless-steel trays and dried in the sun for 6 hours. The samples were scraped from the trays into clean white cotton cloths, wrapped and squeezed. The yield of the oil was calculated using Equation (1), expressed as percentage of the dry weight of the sample [12]. The oils collected were stored in airtight bottles in a functional refrigerator for further use.
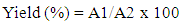 | (1) |
2.3. Characterisation of the Oils
- The cold pressed oils of D. edulis fruits (from Uyo and Ikom) and P. americana fruits were analysed without further purifications for their physicochemical properties viz: freezing point, melting point, pH, smoke point, moisture content, acid value, iodine value and saponification value using standard methods. All the determinations were done in duplicates.
2.3.1. Determination of Moisture Content
- The method described by [13] was used to determine the moisture content of the oils. A well labelled crucible was dried in an oven at 105°C. The crucible was allowed to cool in a dessicator and weighed to a constant weight. Each of the oils (2 g) was added to the crucible, the weight of the crucible with the oil was taken and dried in an oven at 105°C. The crucible with the oil after drying was cooled in a dessicator and weighed to a constant weight. Percentage moisture content was calculated using Equation (2).
 | (2) |
2.3.2. Determination of Freezing Point
- The oil (10 cm3) in a test tube was inserted in a cup containing ice blocks, the temperature at which the oil began to freeze was recorded using a thermometer [14].
2.3.3. Determination of Melting Point
- The melting point of the oil was determined by the method described by [13]. The oil (10 cm3) in a test tube was inserted in a cup containing ice blocks and left to solidify. The solidified oil in the test tube was removed from the ice block, the temperature at which the oil began to melt was recorded using a thermometer.
2.3.4. Determination of Smoke Point
- The oil (10 cm3) was measured into a clean dried crucible and heated on a hot plate. The temperature at which the oil started to form a bluish smoke was recorded using a thermometer [15].
2.3.5. Determination of pH of oil
- The oil (50 cm3) was measured into a clean dried beaker and the probe of a digital pocket-sized pH meter was introduced into the oil and pH recorded at room temperature [16].
2.3.6. Determination of Acid Value (AV)
- The acid value of the oils was determined by using ASTM D465-05 standard method [17]. The individual oils (1 g) was measured into separate conical flasks, followed by addition of 25 cm3 of carbon tetrachloride (CCl4). Two (2) drops of phenolphthalein indicator were also added to the mixture. The mixture was titrated with 0.1 M alcoholic potassium hydroxide (KOH) solution until a colour change was obtained. A blank titration was also carried out without the oil. The acid values of the oils were computed using Equation (3).
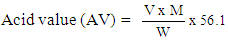 | (3) |
2.3.7. Determination of Saponification Value (SV)
- The ASTM D464-05 standard method [17] was used to determine saponification value. Two grams (2g) of the individual oils were weighed separately in a conical flask and 25 cm3 of 0.5 M ethanolic potassium hydroxide solution was added. Each flask was heated in a steam bath, refluxing for 30 minutes with occasional swirling. The resultant solutions were then titrated with 0.5 M hydrochloric acid (HCl) using 2 drops of phenolphthalein indicator until the pink colour just disappeared. A blank determination, that is, without the oil, was carried out under similar conditions. The saponification values were calculated using the Equation 4.
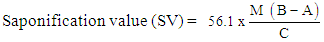 | (4) |
2.3.8. Determination of Iodine Value (IV)
- The ASTM D5768-02 standard method [17] was used to determine the iodine value of the individual oils. To 0.5 g of the individual oils in a separate conical flask, 20 cm3 of carbon tetrachloride was added to the oil. The resultant solution was mixed with 25 cm3 Wijs solution. Each flask with its content were stoppered, swirled to mix, and allowed to stand in the dark for 1 hour at room temperature. Then 20 cm3 of 10% aqueous potassium iodide and 100 cm3 of water were added to the contents in each of the flasks. The content of the flask was titrated with 0.1 M sodium thiosulphate solution until the yellow colour almost disappeared. Starch indicator (1 cm3 of 1%) was added and the titration continued by adding more sodium thiosulphate solution until the blue-black colouration disappeared after vigorous shaking. A blank determination was carried out in the same manner under similar conditions. The iodine value for the oils were determined using (5).
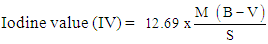 | (5) |
2.3.9. Infrared Spectroscopy (IR)
- Fourier Transform Infrared (FTIR) spectra of the individual oils were obtained using IR Affinity-1S Fourier Transform Infrared spectrophotometer. A horizontal attenuated total reflectance (ATR) sampling accessory equipped with zinc selenide (ZnSe) cell was employed. The cold-pressed oils were used without further purification. Approximately 20 mg of the individual oils were placed in the sampling accessory obtaining the best contact with the crystal. The approximate total time required for spectral collection was 5 min. All spectra were recorded within a range of 4000-650 cm-1 with a 4 cm-1 resolution. Analyses were performed in dry atmosphere (18 ± 0.5°C). Each spectrum was calculated as the average of 20 scans and subjected to background subtraction.
2.4. Soap Production and Analysis
- The method of [18] was used for soap production. The individual oils (100 g) of D. edulis-Uyo, D. edulis-Ikom and P. americana respectively were weighed into separate 500 cm3 beakers, heated to about 100°C and saponification was initiated by adding 20 cm3 of 23.5% sodium hydroxide (NaOH) solution. To the resulting solution, 60 g of NaOH pellets dissolved in 100 cm3 of deionised water was added gradually while stirring until completion of saponification. NaCl (8 g) dissolved in 30 cm3 of deionised water was added to grain soap. The salt was added to separate the spent lye in the bottom, while saponified mass floats on the surface to reduce the soap viscosity and to separate the glycerol water in the bottom. The glycerol water was removed by siphoning. The soap paste was washed with 10 cm3of hot water (90°C) to reduce excess sodium hydroxide and sodium chloride and any impurities found in the soap paste. The soap obtained was washed again with 10 cm3 of distilled water, filtered using a linen cloth, then a small amount of water was added to soften it whilst heating. The soap was placed in a mould and allowed to dry.
2.4.1. Determination of Total Fatty Matter (TFM)
- Total fatty matter of individual soap produced was determined using the method of [18]. Each soap (10 g) was weighed in a beaker and 150 cm3 of deionised water, 20 cm3 of 15% tetraoxosulphate (VI) acid (H2SO4) solution were added. The mixture was heated until the soap dissolved to form a clear solution. Fatty acid on the surface of the resulting solution was solidified by adding 7 g of candle wax and reheated. The solution was allowed to cool to form a cake on the surface of the solution. The cake was removed and left at room temperature to dry to a constant weight. The cake was weighed to obtain the total fatty matter using the Equation (6).
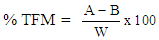 | (6) |
2.4.2. Determination of Free Caustic Alkali
- Free caustic alkali was determined using the method of [18]. Produced soap (5 g) was weighed into a conical flask and 30 cm3 of ethanol was added and the mixture heated until a clear solution was obtained. Barium chloride (10 cm3 of 20%) was added and the solution turned cloudy milky, which later turned pink with addition of few drops of phenolphthalein indicator. The resulting solution was titrated against 0.05 M H2SO4 solution. Free caustic alkali was calculated using Equation (7).
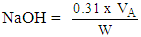 | (7) |
2.4.3. Determination of Moisture Content
- A crucible was dried in an oven at 105°C to a constant weight. The crucible was removed and cooled in a desiccator. The produced soap (3 g) was accurately weighed using analytical balance into the dried crucible and dried in an oven at 105°C and cooled in a desiccator to a constant weight [18]. The % moisture content was calculated using Equation (8)
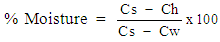 | (8) |
2.4.4. Determination of pH of Soap
- The soap (2.0 g) was weighed into a clean beaker, 20 cm3 of water was added to dissolve the soap in order to prepare 10% aqueous solution of soap. The pH of 10% aqueous solution of the soap was measured by using a pocket-sized digital pH meter at room temperature [19].
3. Results and Discussion
- The physicochemical properties of oil from D. edulis-Uyo, D. edulis-Ikom and P. americana fruits namely: yield, freezing point, melting point, pH, smoke point, moisture content, acid value, iodine value and saponification value are shown in Table 1.
|
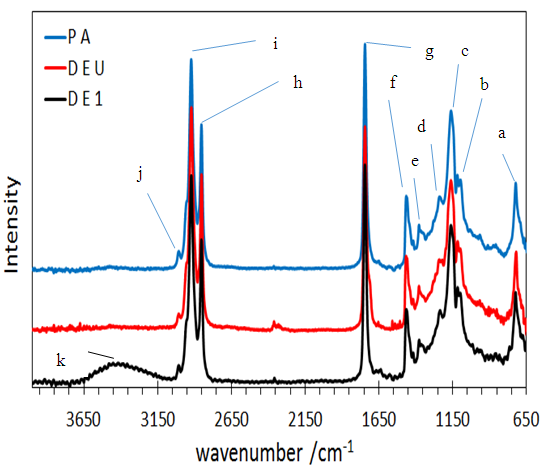 | Figure 2. FTIR of P. americana (P A), D. edulis-Uyo (D E U) and D. edulis-Ikom (D E I) |
|
|
4. Conclusions
- Cold pressed oils extracted from D. edulis-Uyo, D. edulis-Ikom and P. americana fruits could be classified as non-drying oils and could be used as emollients in cosmeceuticals. The higher oil yield of the fruits of D. edulis than P. americana, with similar functional groups (C-HCH3, C=O, C-O, C-HCH2 and -CH=CH), could project D. edulis oils as possible substitute for P. americana oil in cosmeceutical formulations. Comparatively, D. edulis-Uyo oil soap with lowest moisture content (10.33 %), lowest pH (10.1), highest Total fatty matter (89.2%) and free alkaline of 0.07, was the best quality soap produced from the three oils. This was followed by P. americana oil soap with D. edulis-Ikom oil soap having the least quality.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML