-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2019; 9(1): 1-12
doi:10.5923/j.chemistry.20190901.01

Volumetric and Acoustic Properties for Binary Mixtures of N,N-Dimethylformamide with 2-Butanol and 2-Pentanol at Temperatures between 298.15 K and 318.15 K
Aklima Jahan1, Md. Ashraful Alam2, Md. Rabiul Awual3, Shamim Akhtar1
1Department of Chemistry, University of Chittagong, Chittagong, Bangladesh
2Department of Chemistry and Bioengineering, Iwate University, Morioka, Japan
3RENESA, 3-3-22 Sakuraguchi, Kobe-shi, Hyogo, Japan
Correspondence to: Aklima Jahan, Department of Chemistry, University of Chittagong, Chittagong, Bangladesh.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The volumetric and acoustic properties for the binary mixtures of N,N-dimethylformamide with two polar solvents 2-butanol and 2-pentanol have been measured over the entire range of composition at T= (298.15, 303.15, 308.15, 313.15 and 318.15) K and at atmospheric pressure. From these experimental data, for both the systems, excess molar volume, VE, versus mole fraction, x1, of N,N-dimethylformamide curves were calculated and found as sigmoid at all temperatures. The partial molar volume of 2- butanol changes almost rapidly but tends to form a hump like in maximum and minimum mole fraction at different temperatures. Excess isentropic compressibility, KSE, values were negative for both the systems over the entire composition range. All the excess parameter values were fitted using the Redlich-Kister polynomial smoothing equation. In addition, the results were analyzed in terms of molecular interactions and structural effects.
Keywords: 2-Butanol, N,N-Dimethylformamide, Excess Molar Volume, Excess Isentropic Compressibility, 2-Pentanol, Molecular interactions
Cite this paper: Aklima Jahan, Md. Ashraful Alam, Md. Rabiul Awual, Shamim Akhtar, Volumetric and Acoustic Properties for Binary Mixtures of N,N-Dimethylformamide with 2-Butanol and 2-Pentanol at Temperatures between 298.15 K and 318.15 K, American Journal of Chemistry, Vol. 9 No. 1, 2019, pp. 1-12. doi: 10.5923/j.chemistry.20190901.01.
Article Outline
1. Introduction
- The volumetric and acoustic properties of binary mixtures would be a great importance in processing the engineering designs and also helpful in getting information about the molecular structure and intermolecular forces in liquid mixtures, which can be very helpful in making the choice of solvent in various applications [1, 2]. The thermodynamic properties of binary liquid mixtures containing protic, aprotic, and associated liquids have been studied previously [3-7].N,N-dimethylformamide (DMF) is a colorless, non-hydrogen bonded, high-boiling, mobile, highly polar liquid with a faint, characteristic odor. DMF does not decompose at distilling at low pressure and is freely miscible with water, alcohols, ethers, ketones, esters, carbon disulfide, and chlorinated and aromatic hydrocarbons. Molecular interactions of DMF with some solvents reported by various thermodynamic and thermophysical measurements [8]. DMF is aprotic and unassociated [9] in its pure liquid state. It belongs to the so-called super solvents, owing to its miscibility with almost all common polar and no polar solvents [10], probably due to its high polarity with large dipole moment (μ = 3.8D) and moderately high dielectric constant (ε = 36.76) [11]. Several topics and examples of thermodynamic studies are depicted on the basis of the structural behavior of DMF for binary mixtures of non-electrolytes [12].Alkanols are interesting versatile solvents, used in chemical and technological processes which are inexpensive and easily available at high purity. These polar liquids are self-associated through hydrogen bonding, creating multimers of different degrees [13, 14] whether association is disturbed when they are mixed with another solvent [15]. Alkanols are the -OH functional group containing compounds bonded to a carbon atom. Alkanols of short chain length have a greater proton-donor capacity, so the strength of bonding is expected to decrease with an increase in their chain lengths. Moreover, because of the steric hindrance of alkyl groups, hydrogen bonds are weakened for higher length alkanols [12, 16]. Alkanols are also of interest in their own right and serve as simple examples of biologically and industrially important amphiphilic materials [17]. Additionally, these solvents (amides and alkanols) and their mixtures are used as reaction solvents and find applications in many chemical and industrial processes [18].Recently, the substantial research work has been reported on the excess properties of N, N-dimethylformamide +2-alkanols [19], formamide+2-alkanols [20], N,N-dimethylformamide+1-alkanols [21-25] acetophenone +2-alkanols [26] ethylmethylketone+2-alkanols [27], N, N-dimethylaniline+1-alkanols,+2-alkanols,+2-methyl-1-propanol,+2-methyl-2-propanol [28]. Though a number of studies have been carried out on the physical properties of DMF, the reported results are not so sufficient with the existence of some discrepancies. The study of this work is a part of an ongoing research effort to measure and characterize the binary mixtures containing 2-alkanols [29, 30].The densities, ultrasonic velocity and excess properties of the binary blends of these compounds have important impact on the injection process. In order to gain a better understanding of the competitive role of H-bonds associations, the nature and dynamics of the molecular structures in these mixtures, we report the consequences of volumetric and acoustic investigations of 2-butanol and 2-pentanol within the temperature range at T= (298.15, 303.15, 308.15, 313.15 and 318.15) and at atmospheric pressure.Hence, in this paper, we reported the volumetric properties like densities, ρ, excess molar volume, VE, partial molar volume, V, and acoustic properties like ultrasonic velocity, u, excess ultrasonic velocity, uE, excess isentropic compressibility, KSE, excess acoustic impedance, ZE, of the binary systems of N,N-dimethylformamide with two positional isomeric alkanols, such as, 2-butanol and 2-pentanol. These results have been used to discuss the nature of interaction between unlike molecules in terms of hydrogen bonding, dipole-dipole interaction, proton-acceptor interaction and dispersive forces, and also used to analyze the effect of branching in the alkanol and position of the hydroxyl group in the interaction with the N,N-dimethylformamide. Previously, a large number of researchers have demonstrated that in addition to dipole-dipole and Van der Waals interactions, alcohols are strongly self-associated through H-bonding (O-H•••••O-H) interaction. As the liquid they form clusters or networks with restricted rotations about the H-bonds, and hence show variable degrees of polymeric aggregates. The molecular interactions in the present mixtures are also controlled through the formation of the hydrogen bonds of the type C=O•••••H-O, between unlike molecules [31]. In addition, the effects of difference in shape and steric factors on molecular interactions were observed. The results of the present work were found very much similar with the literatures published previously with a few differences. The findings of this study can be utilized in having a better insight into molecular interactions between the components of the systems. It also helps in understanding the biological systems, synthesis of various compounds, process designing in chemical, petrochemical, pharmaceutical industries, paints, inks as well as in other prospects.
2. Experimental Section
2.1. Materials and Method
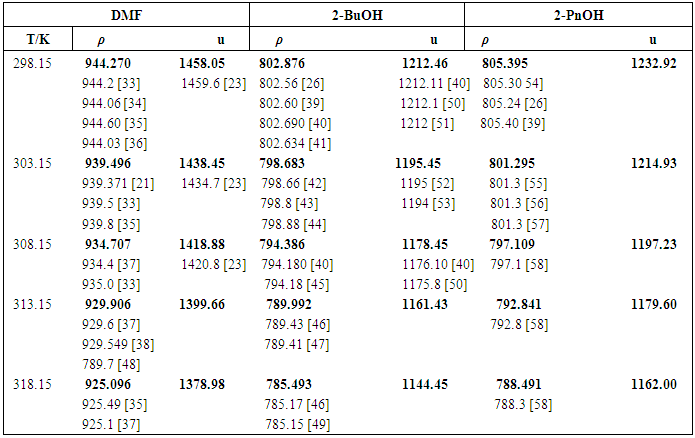
- DMF (Aldrich, purity HPLC grade 99.9+ %), 2-BuOH (Aldrich, purity 99+%) and 2-PnOH (Aldrich, purity 98%) were used without further treatment which is shown in Fig. 1. The densities and ultrasonic velocity of pure chemicals were compared with literature values, which show satisfactory agreements as shown in Table 1.
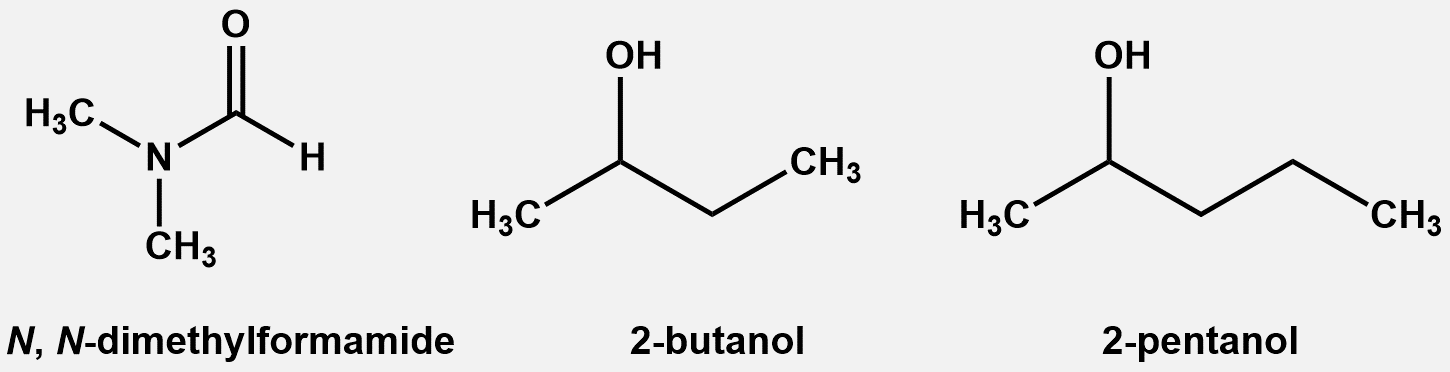 | Figure 1. Chemical structures of the compounds used in the experiments |
3. Results and Discussion
3.1. Densities and Excess Molar Volumes
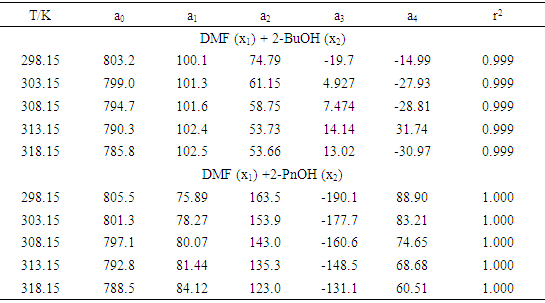
- The experimental results of the density, ρ, measurements of binary mixtures of DMF with 2-BuOH, and DMF with 2-PnOH as a common component, over the whole composition range expressed as mole fractions, x1, of DMF (0 ≤ x1 ≤ 1) at different temperatures are listed in Table 2. The excess molar volumes, VE, were calculated by using the following relation
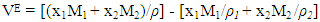 | (1) |
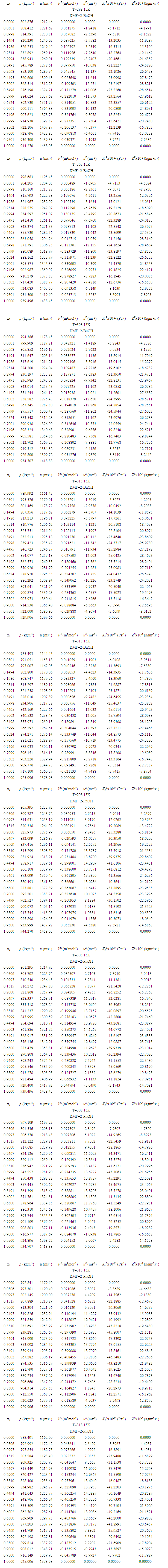 | Table 2. Experimental densities (ρ), excess molar volume (VE), ultrasonic velocity (u), excess ultrasonic velocity (uE), excess isentropic compressibility values (KSE), acoustical impedance (ZE) of the systems DMF (x1) + 2-BuOH (x2) and + 2-PnOH (x2) for different molar ratios at different temperatures |
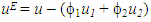 | (2) |
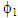 and
and 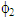 , u1 and u2 are the volume fraction and ultrasonic velocity of component 1 and component 2, respectively.Excess values of acoustic impedance, (ZE) were calculated by the following equation:
, u1 and u2 are the volume fraction and ultrasonic velocity of component 1 and component 2, respectively.Excess values of acoustic impedance, (ZE) were calculated by the following equation: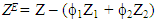 | (3) |
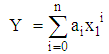 | (4) |
|
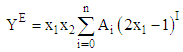 | (5) |
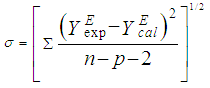 | (6) |
|
|
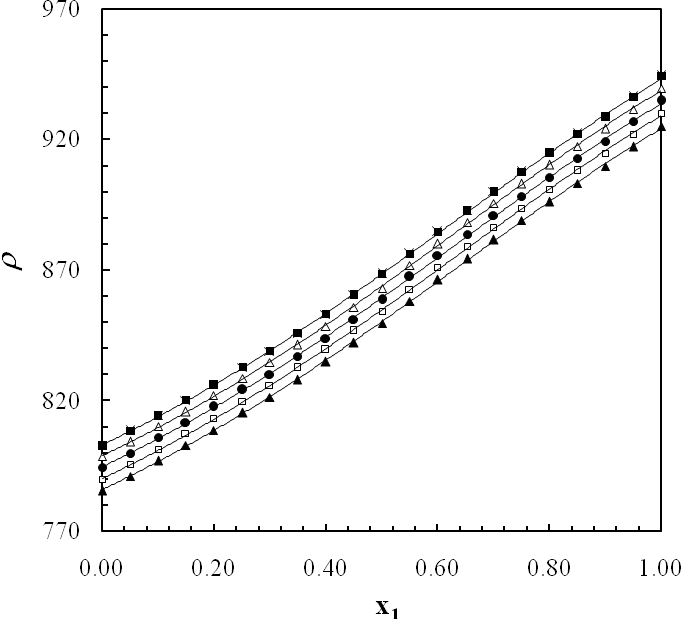 | Figure 2. Density (ρ) of DMF(x1) + 2-BuOH(x2) system for different molar ratios at different temperatures |
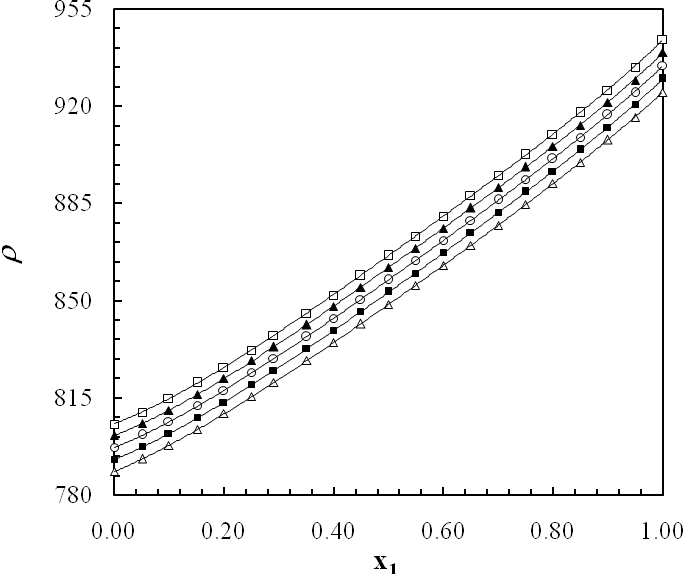 | Figure 3. Density (ρ) of DMF(x1) + 2-PnOH(x2) system for different molar ratios at different temperatures |
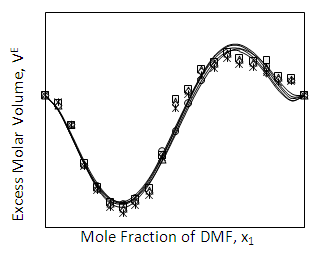 | Figure 4. Excess Molar Volume (VE) of DMF (x1) + 2-BuOH (x2) system for different molar ratios at different temperatures |
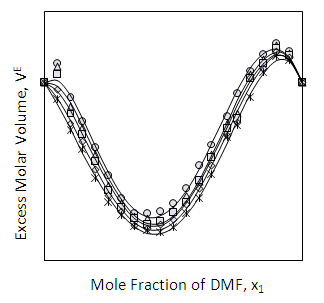 | Figure 5. Excess Molar Volume (VE) of DMF (x1) + 2-PnOH (x2) system for different molar ratios at different temperatures |
3.2. Partial Molar Volumes
- In addition to other volumetric properties, partial molar volumes (
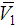 and
and 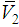 ) and excess partial molar volumes (
) and excess partial molar volumes (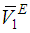 and
and 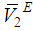 ), of DMF, alkanols over the entire composition range were determined using Equation (7) and Equation (8)
), of DMF, alkanols over the entire composition range were determined using Equation (7) and Equation (8)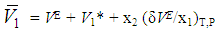 | (7) |
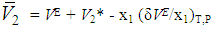 | (8) |
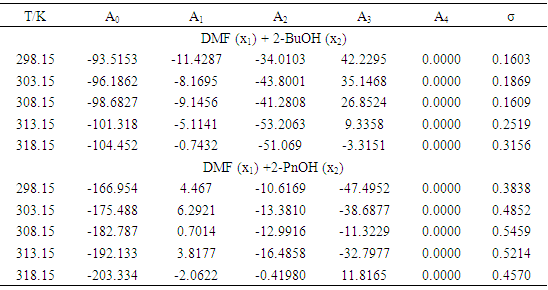
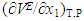 were obtained.Accordingly, by differentiating Equation (5) for VE with respect to x1 and x2 subsequently, substituting of its value in Equation (7) and Equation (8) lead to the following Equation (9) and Equation (10):
were obtained.Accordingly, by differentiating Equation (5) for VE with respect to x1 and x2 subsequently, substituting of its value in Equation (7) and Equation (8) lead to the following Equation (9) and Equation (10):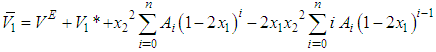 | (9) |
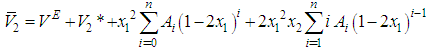 | (10) |
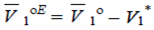 | (11) |
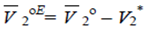 | (12) |
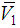 of 2-PnOH was slightly risen in the DMF-poor region, but rapidly decreased in the DMF-rich region at lower temperatures. Considering, the significances of
of 2-PnOH was slightly risen in the DMF-poor region, but rapidly decreased in the DMF-rich region at lower temperatures. Considering, the significances of 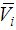 , the falling
, the falling 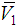 of 2-PnOH and also to form the minimum at x2 ≈ 0.85 reveals that in the overall volume expansion, the alkanol contributes slightly but in contraction 2-PnOH contributes quite significantly making the corresponding VE values negative in the DMF-rich region. This is just in accordance to the result that, in the stated region above (x2 = 0.30) the value of (V1 – V1) falls to be negative with minimum near x2 = 0.80 for the system of 2-PnOH + DMF. Likewise, the partial molar volume of 2-BuOH (
of 2-PnOH and also to form the minimum at x2 ≈ 0.85 reveals that in the overall volume expansion, the alkanol contributes slightly but in contraction 2-PnOH contributes quite significantly making the corresponding VE values negative in the DMF-rich region. This is just in accordance to the result that, in the stated region above (x2 = 0.30) the value of (V1 – V1) falls to be negative with minimum near x2 = 0.80 for the system of 2-PnOH + DMF. Likewise, the partial molar volume of 2-BuOH (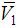 ) though apparently changes very small in the whole range of concentration, (
) though apparently changes very small in the whole range of concentration, ( – V1) values are all quite large, being positive in the region 0 < x2 < 0.65 and sharp negative above x2 > 0.65. This clearly signifies that, contribution of 2-BuOH towards VE is positive in the former region, while it is negative above x2 > 0.65. However, the magnitudes of VE, whether positive or negative are greater for 2-BuOH + DMF than that for 2-PnOH + DMF.
– V1) values are all quite large, being positive in the region 0 < x2 < 0.65 and sharp negative above x2 > 0.65. This clearly signifies that, contribution of 2-BuOH towards VE is positive in the former region, while it is negative above x2 > 0.65. However, the magnitudes of VE, whether positive or negative are greater for 2-BuOH + DMF than that for 2-PnOH + DMF. | Figure 6. Partial molar volume 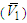 of 2-BuOH in 2-BuOH(x2) + DMF(x1) system for different molar ratios at different temperatures of 2-BuOH in 2-BuOH(x2) + DMF(x1) system for different molar ratios at different temperatures |
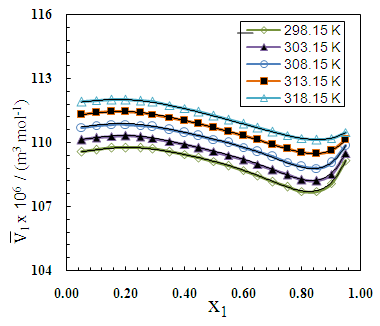 | Figure 7. Partial molar volume 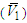 of 2-PnOH in 2-PnOH (x2) + DMF (x1) system for different molar ratios at different temperatures of 2-PnOH in 2-PnOH (x2) + DMF (x1) system for different molar ratios at different temperatures |
3.3. Excess Isentropic Compressibility
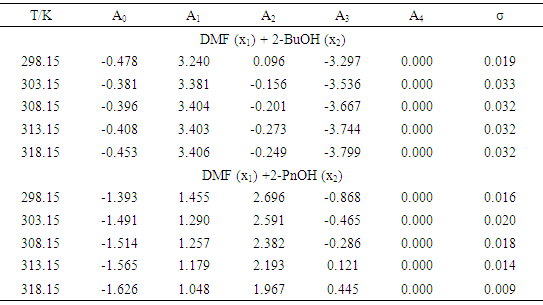
- The isentropic compressibility, KS, was calculated from density, ρ, and ultrasonic velocity, u, assuming that ultrasonic absorption is negligible, using the Newton-Laplace equation, KS =1/ρu2.Then, the excess isentropic compressibility, KSE, (±10−1 TPa−1) can be determined according to the following equation:
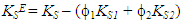 | (13) |
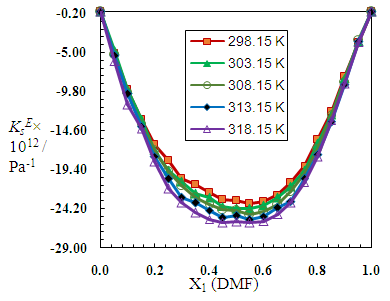 | Figure 8. Excess isentropic compressibilities (KsE), for N,N-dimethylformamide (X2) + 2-BuOH (X1) system at different molar ratios at different temperatures |
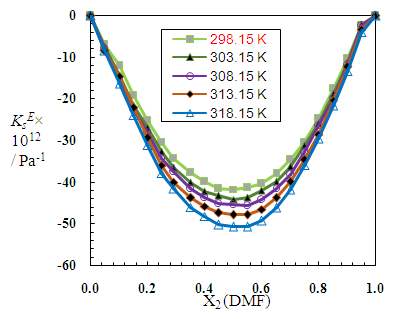 | Figure 9. Excess isentropic compressibilities, (KsE), for N,N-dimethylformamide (X2) + 2-PnOH (X1) system at different molar ratios at different temperatures |
4. Conclusions
- The density, ultrasonic velocity and some excess volumetric and acoustic properties of binary mixtures of N,N dimethylformamide with 2-butanol and 2-pentanol have been measured over the entire range of composition at T= (298.15, 303.15, 308.15, 313.15 and 318.15) K and at atmospheric pressure. The Redlich-Kister polynomial equation was used to correlate the results. From the experimental data, the positive VE suggests that, DMF-alkanol interaction (structure making effect) is weaker than that of DMF-DMF and alkanol-alkanol interactions (structure breaking effect) and vice-versa for the negative VE. Dissociation of H-bonded structures of 2-alkanols results into positive VE, whereas, dissociation/formation of weak H-bonds between DMF and 2-alkanols leads to negative VE. In applications, this study would be helpful in understanding the physical properties as well as chemical properties of mixtures between DMF and other solvents.
ACKNOWLEDGEMENTS
- The authors are grateful to the Department of Chemistry, University of Chittagong, Chittagong-4331, Bangladesh.
References
| [1] | Gupta M., Vibhu I., Shukla J.P.: 2006. Fluid Phase Equilib. 244, 26-32. |
| [2] | Almasi M.: 2014. J. Chem. Thermodynamics 69, 101-106. |
| [3] | Kim K.S., Marsh K.N.: 1988. J. Chem. Eng. Data 33, 288-292. |
| [4] | Nayak J.N., Aralaguppi M.I., Aminabhavi T.M.: 2001. J. Chem. Eng. Data 46, 891-896. |
| [5] | Henni A., Tontiwachwathikul P., Chakma A., Mather A.E.: 1999. J. Chem. Eng. Data 44, 101-107. |
| [6] | Ali A., Nain A.K.: 2001. Indian J. Pure Appl. Phys. 39, 421-427. |
| [7] | Pal A., Bhardwaj R.K.: 2001. J. Indian Chem. Soc. 78, 18-22. |
| [8] | Almasi M.: 2014. Journal of the Taiwan Institute of Chemical Engineers 45, 365-371. |
| [9] | Shuqin L., Xingen H., Ruisen L.: 1999. J. Chem. Eng. Data 44, 353-356. |
| [10] | Krestov G.A.: 1991. Thermodynamics of Solvation; Eills Harwood: England. |
| [11] | Marcus Y.: 1977. Introduction to Liquid-State Chemistry, Wiley-Inter-science: London. |
| [12] | Venkatesu P.: 2010. Fluid Phase Equilib. 298, 173-191. |
| [13] | Karlapudi S., Gardas R.L., Venkateswarlu P., Sivakumar K.: 2013. J. Chem. Thermodynamics 67, 203-209. |
| [14] | Tsierkezos N.G., Palaiologou M.M., Molinou I.E.: 2000. J. Chem. Eng. Data 45, 272-275. |
| [15] | Mrad S., Hichri M., Khattech I., Lafuente C.: 2017. J. Mol. Liq. 231, 168-173. |
| [16] | Schore N.E., Vollhardt K.P.C.: 2007. Organic chemistry: structure and function. New York: W.H. Freeman and Co. |
| [17] | Yang C., Liu Z., Lei H., Ma P.: 2006. J. Chem. Eng. Data 51, 6653-6661. |
| [18] | Iloukhani H., Ghorbani R.: 1998. J. Solution Chem. 27, 1790-1796. |
| [19] | Almasi M.: 2014. J. Chem. Eng. Data 59, 275-281. |
| [20] | Almasi M.: 2014. J. Chem. Thermodynamics 69, 101-106. |
| [21] | Thirumaran S, Mathammal R, Thenmozhi P. Chem. Sci. Trans. 2012;1:674-682. |
| [22] | Thirumaran S., Alli T., Priya D., Selve A.: 2010. E-Journal of Chemistry 7, 217-222. |
| [23] | Kannappan A.N., Thirumaran S., Palani R.: 2009. Journal of Physical Science 20, 97-108. |
| [24] | Tuwaim M.S.A., Alkhaldi K.H.A.E., Al-Jimaz A.S., Mohammad A.A.: 2012. J. Chem. Thermodynamics 48, 39-47. |
| [25] | Thirumaran S., Sabu K.J.: 2012. International Journal of Recent Scientific Research 3, 627- 636. |
| [26] | Almasi M., Iloukhani H.: 2010. J. Chem. Eng. Data 55, 1416-1420. |
| [27] | Almasi M.: 2013. Thermochimica Acta 554, 25-31. |
| [28] | Manukonda G., Ponneri V., Kasibhatta S., Sakamuri S.: 2013. Korean J. Chem. Eng. 30, 1131-1141. |
| [29] | Iloukhani H., Samiey B., Moghaddasi M.A.: 2006. J. Chem. Thermodynamics 38, 190-200. |
| [30] | Iloukhani H., Parsa J.B., Saboury A.A.: 2000. J. Chem. Eng. Data 45, 1016-1018. |
| [31] | Rao K.P., Reddy K.S.: 1985. Thermochimica Acta 91, 321-327. |
| [32] | International Union of Pure and Applied Chemistry.: 1986. Inorganic Chemistry Division, Commission on Atomic Weights and Isotropic Abundances. Pure appl. Chem. 58, 1677-1692. |
| [33] | Baragi J.G., Aralaguppi M.I., Aminabhavi T.M., Kariduraganavar M.Y., Kittur A.S.: 2005. J. Chem. Eng. Data 50, 910-916. |
| [34] | Garcı´a B., Alcalde R., Leal J.M.: 1997. J. Phys. Chem. 101, 7991-7997. |
| [35] | Bernal-García J.M., Guzmán-López A., Cabrales-Torres A., Estrada-Baltazar A., Iglesias-Silva G.A.: 2008. J. Chem. Eng. Data 53, 1024-1027. |
| [36] | Tong-Chun B., Jia Y., Shi-Jun H.: 1999. J. Chem. Eng. Data 44, 491-496. |
| [37] | Akhtar S., Omar Faruk A.N.M., Saleh M.A.: 2001. Phys. Chem. Liq. 39, 383-399. |
| [38] | Sharlin P., Steinby K., Doman´ska U.: 2002. J. Chem. Thermodynamics 34, 927-957. |
| [39] | Riddick J.A., Bunger W.B., Sakano T.K.: 1986. Organic Solvents, Physical Properties and Methods of Purification, 4th ed. John Wiley, Sons: New York. |
| [40] | Kumar H., Kaur M., Gaba R., Kaur K.: 2011. J. Therm. Anal. Calorim. 105, 1071-1080. |
| [41] | Peralta R.D., Infante R., Cortez G., Cisneros A., Wisniak W.: 2005. J. Chem. Eng. Commun. 192, 1684-94. |
| [42] | Awwad A.M., Alsyouri H.A., Jbara K.A.: 2008. J. Chem. Eng. Data 53, 1655-1659. |
| [43] | Ali A., Nain A.K., Lal B., Chand D.: 2004. International Journal of Thermophysics 25, 1835-1847. |
| [44] | Rao K.P., Reddy K. S.: 1985. Thermochimica Acta 91, 321-327. |
| [45] | Lomte S.B., Bawa M.J., Lande M.K., Arbad B.R.: 2009. J. Chem. Eng. Data 54, 127-130. |
| [46] | Nain A.K.: 2007. J. Solution Chem. 36, 497-516. |
| [47] | Giner B., Artigas H., Carrion A., Lafuente C., Royo F.M.: 2003. J. Mol. Liq. 8, 303-311. |
| [48] | TRC Thermodynamic Tables, Non-Hydrocarbons, Thermodynamics Research Center, The Texas A, M University System: College Station, TX. 1993. |
| [49] | Anson A., Garriga R., Martinez S., Perez P., Gracia M.: 2005. J. Chem. Eng. Data 50, 677-682. |
| [50] | Outcalt S.L., Laesecke A., Fortin T.J.: 2010. J. Mol. Liq. 51, 50-59. |
| [51] | Gonzalez B., Dominguez A., Tojo J.: 2006. J. Chem. Thermodynamics 38, 1172-1185. |
| [52] | Babu K.R., Venkateswarlu R., Raman G.K.: 1989. Asian J. Chem. 1, 147-151. |
| [53] | Venkatesulu D., Venkatesu P., Prabhakara M.V.R.: 1997. J. Chem. Eng. Data 42, 1145-1146. |
| [54] | Almasi M., Iloukhani H.: 2010. J. Chem. Eng. Data 55, 3918-3922. |
| [55] | Wen-Lu W., Liang-Tau C., Min S.I.: 1999. J. Chem. Eng. Data 44, 994-997. |
| [56] | TRC Thermodynamic Tables, Non-Hydrocarbons, Thermodynamics Research Center, The Texas A&M University System: College Station, TX. 1994. |
| [57] | Wen-Lu W., Yule-Chen C., Chu-Ping H.: 1999. J. Chem. Eng. Data 44, 998-1001. |
| [58] | Akhtar S., Hossain K.M.S., Saleh M.A.: 2002. Physics and Chemistry of Liquids 40, 435-448. |
| [59] | Redlich O., Kister A.T.: 1948. Ind. Eng. Chem. 44, 345-348. |
| [60] | Maham Y., Teng T.T., Hepler L.G., Mather A.E.: 1994. J. Sol. Chem. 23, 195-205. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML