-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2018; 8(5): 114-117
doi:10.5923/j.chemistry.20180805.03

Green Pre-treatment of Cotton Fabric Inwardly Eco-friendly Way
Md. Mehedi Hasan Rubel1, Mohammad Arifur Rahman2, Md. Mazharul Islam2, Jamal Haider Hasib1, Mofasser Haque Chowdhury1
1School of Textile Science and Engineering, Wuhan Textile University, Wuhan, China
2School of Mechanical Engineering and Automation, Wuhan Textile University, Wuhan, China
Correspondence to: Md. Mehedi Hasan Rubel, School of Textile Science and Engineering, Wuhan Textile University, Wuhan, China.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

A facile approach of Hydrogen Peroxide and Irradiation of doses from Cobalt-60 gamma the source is applied to 100% cotton fabric pretreatment reported in this study. Novel changes are observed in Absorbency, Whiteness Index, Bursting Strength and Weight Loss percentage. Various concentration of H2O2 treated different samples absorbed different dosed of Co-60 gamma by the decay of fabric surface to give shiny effect on it as pretreatment replaces of scouring and bleaching. Reduce the use of lots of water and hazardous chemicals of conventional which has novel benefits for the environment. This less time-consuming process makes more efficient comparatively conventional method.
Keywords: Pretreatment, Hydrogen Peroxide, Irradiation, Dosed absorbed
Cite this paper: Md. Mehedi Hasan Rubel, Mohammad Arifur Rahman, Md. Mazharul Islam, Jamal Haider Hasib, Mofasser Haque Chowdhury, Green Pre-treatment of Cotton Fabric Inwardly Eco-friendly Way, American Journal of Chemistry, Vol. 8 No. 5, 2018, pp. 114-117. doi: 10.5923/j.chemistry.20180805.03.
Article Outline
1. Introduction
- Cotton contains about 90% of cellulose and around 10% of non-cellulosic substances. As Cotton is too great extent developed cellulose, around 87-90%, so it is a hardened material and high in rigidity. These cellulosic and non-cellulosic pollutions incorporate waxes, pectin's, proteins, fats and fiery remains which are mostly on the cuticle and primary wall of the fiber. [1] For the activeness of these substances in the fiber, cotton is hydrophobic. That is the reason broad cleaning process is required to make the fiber hydrophilic. The conventional scouring and bleaching procedure of expelling these polluting influences makes incredible damage the earth and in addition, causes additional treatment of gushing. The distinctive elective answer for eco-friendly cotton preparing is trialed however the craving arrangement still unrealistic to reach. [2] We attempted to treat the cotton fabric by gamma radiation for scouring and bleaching. The properties, for example, Whiteness Index (WI), weight reduction, blasting quality, absorbency are assessed. The Whiteness Index of treated fabric increments with the expansion of a portion of the radiation and concentration of H2O2. In spite of the fact that the bursting strength is higher than the conventional sample yet with the expansion of H2O2 concentration, the strength is lower. In spite of the fact that the best absorbency did not accomplish but rather the absorbency test results demonstrates that it is conceivable by gamma radiation to treat cotton texture more eco-friendly. The weight reduction percentage of the treated fabric demonstrates that the radiation changes the surface structure of cellulose creating hydrocellulose and oxy-cellulose. [3] It is certain that radiation innovation can be one of the options for hazardous cotton preparing.
2. Experimental
2.1. Materials
- 100% Cotton 24s/1 yarn was used to manufacture the Twill structure of 220 GSM grey fabric collected from Microfiber Ltd .an export oriented knit composite industries. 7% (w/w) commercial hydrogen peroxide was used in this investigation. This was used without future purification and treatment. A Cobalt-60 gamma source (model gamma 650 no.iir) was used to irradiate the fabric samples according to AATCC Test Method 79-2000 [4]. Truburst strength tester used to measure the tensile (bursting) strength of the samples. The electric balance was used to measure the weight to determine the weight loss.
2.2. Methodology
2.2.1. Sample Preparation
- 100% grey cotton fabric was cut into 10cm x 8cm dimension. There were 25 samples of 5 sets and each set was immersed in different hydrogen peroxide content solution. The amount of hydrogen peroxide solution was 0, 10, 20, 30 and 40 g/L. The samples were immersed for 5 min.
 | Figure 1. Grey Cotton Twill Fabric Saturated with Different Concentration of Hydrogen Peroxide |
2.2.2. Irradiation
- The prepared samples were illuminated at Institute of Radiation and Polymer Technology (IRPT), at Atomic Energy Establishment at Ashuliya, Savar, and Dhaka. The total absorbed doses were 4000, 8000, 12000 and 16000 Gy at a dose rate 60 Gy/min.
2.2.3. Conventional Scouring
- 5g of grey cotton fabric was scoured following to the recipe format.
|
2.2.4. Weight Loss Calculation
- Tests were dried in room temperature. After then the weight was estimated by an electric parity. The weight reduction % of the samples were discovered by the accompanying recipe [5].W % = [(W1 – W2)/W1] X 100, Where, W1 and W2 are the heaviness of the samples when treatment separately.
2.2.5. Absorbency Determination
- The absorbency of various fabric samples was estimated by AATCC test strategy 79-2000. The fabric was mounted in 5-inch width a weaving circle. Refined water was taken in the burette was 2 cm tallness. The weaving loop was set under the burette and one drop of water was permitted to fall on the fabric surface. [6] Amid this time a stopwatch was begun and record the time until the drop of water vanished away. Five drops in better place of the textured surface were taken and the normal time was recorded as the receptiveness.
2.2.6. Whiteness Index (WI) Determination
- The whiteness index of different samples was measured according to AATCC test method 110-1995. The CIE whiteness was measured on a data color with the following setting:Illuminant D65, Large area view, 10-degree observer
2.2.7. Bursting Strength Determination
- The malleable (Bursting) quality of the samples was estimated by BSEN ISO 13938-2:1999 (89). As per this method test sample of 50 cm squire, the diameter was cinched over a broad stomach by methods for around clasping ring. Expanding compacted air is connected to the underside of the stomach causing distension of the stomach and the texture. The weight expanded easily until the point that the test sample blasts. [7] The blasting quality and blasting distension are resolved. The weight of the machine ought to be adjusted to the point that it would blast the sample in 20 ± 5 sec.
3. Results and Discussions
3.1. Analysis of Absorbency
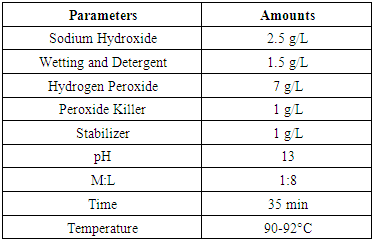
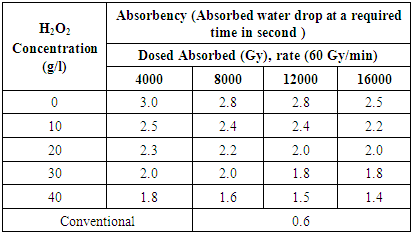
- The absorbency of illuminated samples fluctuates with add up to retained dosage and grouping of Hydrogen Peroxide. The table 2 describes the absorbency at 4000, 8000, 12000, 16000 Gy against 0, 10, 20, 30, 40 g/l Hydrogen Peroxide fixation.
|
3.2. Analysis of Whiteness
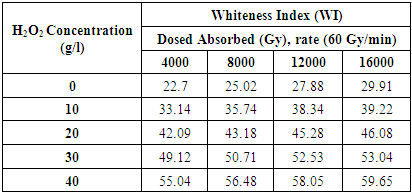
- The CIE whiteness list (WI) of the treated samples increments with an increment of aggregate ingested measurements and concentration of H2O2. The WI esteems at 4000 Gy illuminated samples are 22.7, 33.14, 39.94, 42.09, 47.32, 49.12, 55.04 for 0, 10, 20, 30 and 40 g/l H2O2 concentration separately. The normal shading is much obliterated and the ideal impact is gotten for 10-30 g/l H2O2 concentration. Table 3 express the whiteness record of various retained dosage against various H2O2 concentration.
|
3.3. Analysis of Weight Loss
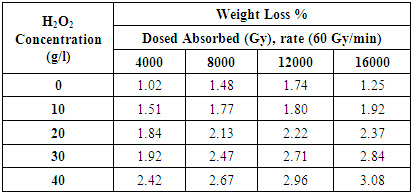
- The weight reduction of dim cotton is the after effect of loss of noncellulose materials from the fabric. Generally traditional soluble scouring with hydrogen peroxide went with 7 to 10% Wight loss, this is for the evacuation of a few hydrocarbons, pectin's, wax etc. in illuminated tests the weight reduction ranges from 1.02 to3.08 for various doses & distinctive hydrogen peroxide centralization of H2O2 such as 0, 10, 20, 30 and 40 g/l. were utilized to examine the scouring & bleaching impact of cotton weaved fabric [8]. When the neighborhood does consume is strengthened the weight reduction % is expanded too. This is valid for expanding the grouping of H2O2 additionally from watched the table 4. It is watched that the weight reduction brings down when samples illuminated without H2O2. The WI % of illuminated of 4000 Gy samples are 1.51, 1.84, 1.92 and 2.42 for 10, 20, 30 and 40 g/l H2O2 respectively. The WL % expanded with the expansion of dosed retained and concentration of hydrogen peroxide. The finding demonstrated the amount of Non-cellulose material expelled from the fabric surface is higher with the activity of higher grouping of H2O2 forthesame radiation does. [9] This wonder watched consistently in all radiation dosed. Another reality is additionally essential that weight loss% of the higher measurements is higher than the comparing lower radiation does from a similar H2O2 concentration.[10] For instance, the estimation of weight reduction at 12000 Gy are 1.74, 1.80, 2.22, 2.71 and 2.96% which is more prominent then 1.48, 1.77, 2.13, 2.47 and 2.67% respectively for the relating 8000 Gy dose with a few exemptions.
|
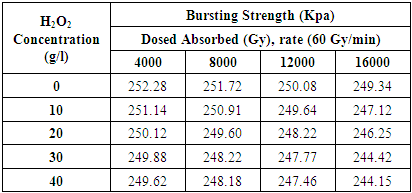
3.4. Analysis of Bursting Strength
- The quality of the samples were estimated by blasting quality analyzer and communicated in KPa with proportionate expansion esteems in mm. For the most part blasting quality connects with the weight loss%. The blasting quality declines with the expansion of aggregate dose assimilated and centralization of hydrogen peroxide. [11] The blasting quality of the sample splashed with 0, 10, 20, 30 and 40 g/l hydrogen peroxide and illuminated at 4000 Gy are 252.28, 251.14, 250.12, 249.88, and 249.62 respectively.
|
4. Conclusions
- The irradiated sample shows good absorbency and weight loss% is less respect to the conventional scoured bleached fabric. The optimum level of absorbency can be obtained by exposing with 4000-16000 Gy total doses. The presence of H2O2 accelerates the absorbency. The sample treated with 8000 Gy plus 20-30 g/l hydrogen peroxide exhibits good absorbency. The water drop is absorbed readily within 2-3 seconds. The whiteness index specifies the bleaching effect of the fabric. The whiter the sample higher is the destruction of natural colors. The natural color of grey cotton is destroyed significantly except the sample of unsaturated with hydrogen peroxide. The other samples all are suitable for pale shade dyeing. By Pretreatment process, we need a lot of water and various chemicals as well as Time at Conventional process but this Experiment makes a revolution for Cotton fabric pretreatment within a short time under the eco-friendly system as well as cost effective this one.
ACKNOWLEDGEMENTS
- We acknowledge the Micro Fiber Ltd. for providing the testing facilities and chemicals as well as also delightful to express gratitude to Chief Scientific Officer and director, IRPT, Bangladesh Atomic Energy Establishment, for his valuable suggestions and guidelines.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML