-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2018; 8(5): 107-113
doi:10.5923/j.chemistry.20180805.02

Chromating of Wool Fibers Dyed with Madder Colorants, Luteolin Colorant and Iron Surface Treatment with a Residual Chromating Bath
Younes Chemchame1, Abdelkhalak Irrou1, Khalid Frah2, Mohamed Tahiri2, El Mehdi Bouchti3
1Department of Traditional Weaving, Academy of Traditional Arts, Foundation of Hassan II Mosque, Casablanca, Morocco
2Department of Metalwork, Academy of Traditional Arts, Foundation of Hassan II Mosque, Casablanca, Morocco
3REMTEX Laboratory, High School of Industry Textile and Clothing (ESITH), Oulfa, Casablanca, Morocco
Correspondence to: Younes Chemchame, Department of Traditional Weaving, Academy of Traditional Arts, Foundation of Hassan II Mosque, Casablanca, Morocco.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The chromating of madder colorants (Rubia tinctorum) and luteolin colorant from dyer’s weed (Reseda luteola) used in wool dyeing allows for a high degree of washing and rubbing fastness. The chromating process was optimized and verified by calculating the fixation rate of natural dyes and the reduced chrome. Chrome-dyes achieved a higher fixation rate than those without chromating dyes (61.8% versus 16.0% for madder colorants and 30.1% versus 11.0% for a luteolin colorant). The residual chromating bath was used to passivate the iron surface to avoid chrome rejection in the environment. The passivation was efficient, and the chrome was used almost completely on the metal surface. The chromating dyeing achieved a rigid fixation, as confirmed by washing-fastness test 105C6A01.
Keywords: Wool, Dyeing, Dyer’s weed, Reseda luteola, Rubia tinctorum, Chromating, Metal passivation, Washing fastness
Cite this paper: Younes Chemchame, Abdelkhalak Irrou, Khalid Frah, Mohamed Tahiri, El Mehdi Bouchti, Chromating of Wool Fibers Dyed with Madder Colorants, Luteolin Colorant and Iron Surface Treatment with a Residual Chromating Bath, American Journal of Chemistry, Vol. 8 No. 5, 2018, pp. 107-113. doi: 10.5923/j.chemistry.20180805.02.
Article Outline
1. Introduction
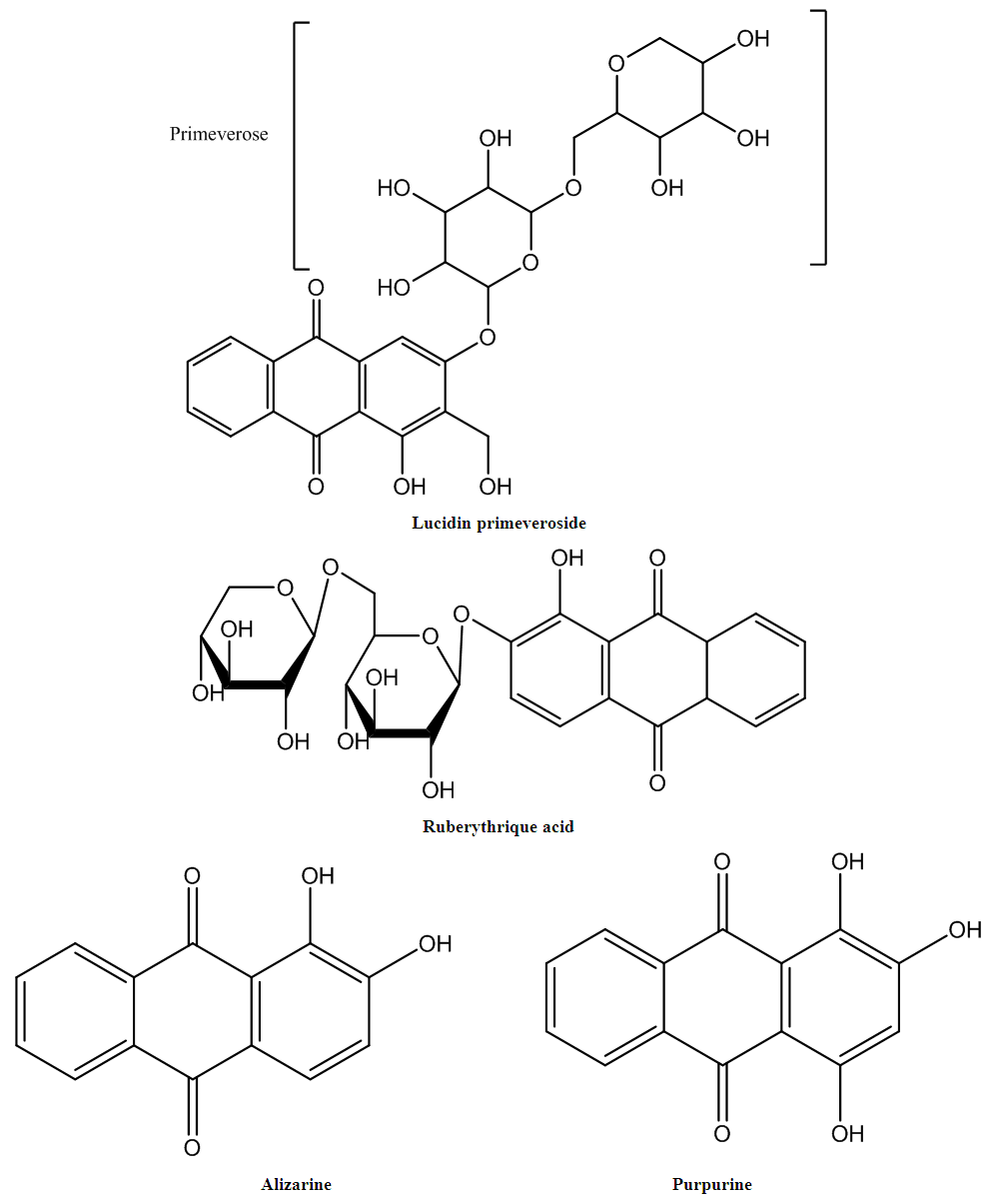
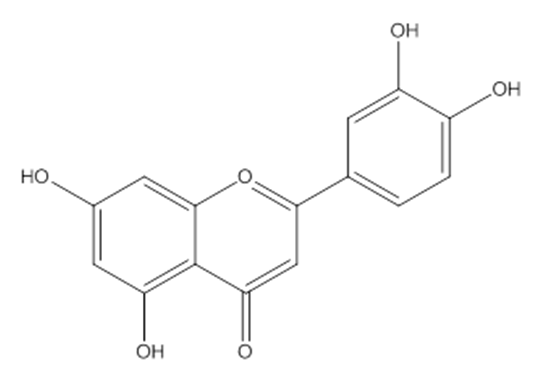
- Mordant dyes have chelating sites that form stable coordination complexes with metal ions from metal salts (mordants). The dye–mordant reaction or chelation requires the presence of salt-forming groups, such as hydroxy or nitroso groups, and the presence of oxygen or nitrogen-containing groups, such as carbonyl, carboxyl, and azo groups, so that a lone-pair electron can be donated to the chromium atom [1]. The dyes used in this study contained oxygen and hydroxy groups in the anthraquinone chemical structure (in dye extracted from the madder plant (Rubia tinctorium)) and in the luteolin chemical structure (in dye extracted from the Reseda luteola plant) (Figures 1 and 2).
 | Figure 1. Chemical structures of principal madder colorants |
 | Figure 2. Chemical structure of Luteolin (dyer’s weed) |
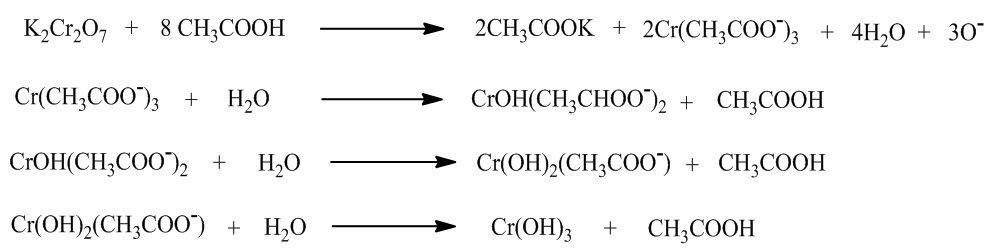 | Figure 3. Reduction reaction of chrome (transformation from the hexavalent chrome VI to the trivalent chrome III) |
2. Experimental
2.1. Materials and Method
2.1.1. Wool Fiber Features
- Wool fiber was from Boujaâd in Morocco. White fleece was compacted and homogenized into a medium-weight fleece of 1.5–3 kg, and the fiber fineness was 50–60 on the Bradford scale [16, 17].
2.1.2. Metal Plaques
- The metal plaques were zinc with dimensions of 60 mm × 40 mm × 4 mm, which were fixed in the anode of the electrolyzer and iron with dimensions of 60 mm × 40 mm × 2 mm, which was fixed at the cathode.
2.1.3. Natural Dye
- The madder colorants (mainly ruberythric acid, lucidin primeveroside, alizarine, and purpurine (Figure 1) [18, 19], and luteolin dye (the main colorant in plants, Figure 2) [20-22] were extracted from the R. tinctorum plant and R. luteola, respectively. These plants grow in the southeast and central regions of Morocco [23]. The extraction method was based on the boiling of a dried and powdered root of the R. tinctorum plant and of the dried stem and leaves of R. luteola.
2.1.4. Chemicals
- The acidic reagents acetic acid (CH3COOH), hydroxide chloride (HCl), nitric acid (HNO3), and the mordant reagent of potassium dichromate (K2Cr2O7) were of analytical grade and were from Sigma Aldrich (Casablanca, Morocco).
2.1.5. Spectrophotometry
- An ultraviolet–visible Thermo Evolution 300 spectrophotometer was used with a spectral bandwidth from 0.2 to 4 nm and double-beam technology, able to operate either offline or under the control of a personal computer, and with a photodiode detector or photomultiplier.
2.1.6. Electrolyzer
- A Combi 5 electrolyzer was coupled electrically to a generator to receive electrical power from 0 to 10 V.
2.1.7. Ph Meter
- A Henne AD1000 pH meter was used as a multimeter to measure pH, oxidation–reduction potential, and temperature of dye bath and dye extraction.
2.1.8. Bath
- A 250-mL flask was used in dyeing and dye extraction. Heating was performed with a thermostat hotplate (Scilogex MS-H280-Pro).
2.1.9. Filter
- The filter was a metallic sieve (1–5-mm diameter).
2.2. Spectral Analysis
2.2.1. Spectrophotometer Calibration
- Spectrophotometer calibration was achieved using a standard solution that was prepared according to the mass of wool yarn and the concentration of every ingredient that was added to the dye or chromating baths.
2.2.2. Measurement of Dye Exhaustion and Fixation Rate
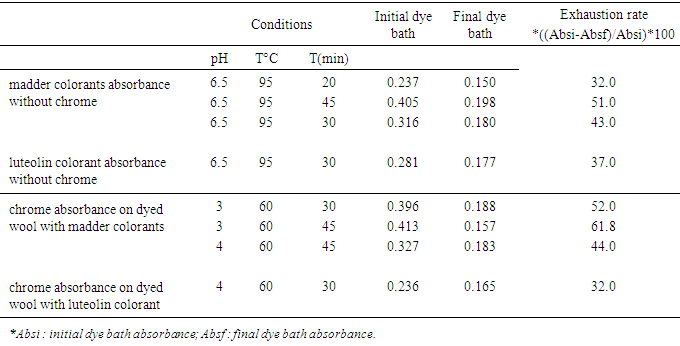
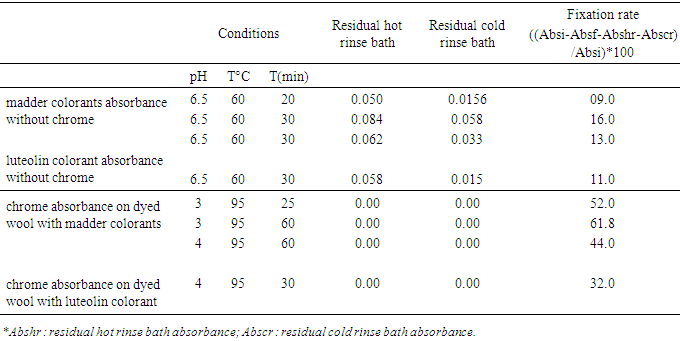
- We removed 1 mL of solution from each dye bath for measurement. Each sample was diluted to 10 mL using the prepared standard solutions. The absorbance measurements are provided in Tables 1 and 2. The absorbance values were measured at 400 nm.
2.3. Dyeing Process with Madder Colorants and Luteolin Dye
2.3.1. Preparation of Madder Dye Bath
- Madder plant samples (10 g) were macerated in 100 mL distilled water at 95°C for 30 min and filtered using a metallic sieve.
2.3.2. Preparation of Luteolin Dye Bath
- R. tinctorum plant samples (14 g) were macerated in 100 mL distilled water at 95°C for 30 min and filtered using a metallic sieve.
2.3.3. Dyeing in the Madder Colorant Dye Bath
- Wool yarn (2 g) was soaked and wrung before being placed in the dye bath. The dyeing conditions of the dye bath were madder colorants (50 mL) at pH 6.5, at 95°C for 20 min, 30 min or 45 min with a liquid ratio of 1/50, according to the dyeing cycle illustrated in Figure 4.
2.3.4. Post Chromating of the Madder-dyed Sample
- Potassium bichromate (1.5 g) and acetic acid (15 mL) at pH 3 or pH 4 were added to the dye bath after the dyed yarn had been removed, and the temperature was decreased to 60°C. A reduction reaction proceeded at this temperature for 30 min or 45 min, as shown in Figure 4.The dyed yarn wool was returned to the chromed dye bath, the temperature was increased to 95°C, and treatment continued for 25 min (Figure 4).
2.3.5. Dyeing in the Luteolin Dye Bath
- Wool yarn (2 g) was soaked and wrung before being placed in the dye bath. The dyeing conditions of the dye bath were luteolin (50 mL) in the dye bath and pH 6.5 at 95°C for 30 min with a liquid ratio of 1/50 (Figure 4).
2.3.6. Post Chromating of the Luteolin Dyed Sample
- Potassium bichromate (0.7 g) and acetic acid (5 mL) at pH 4 were added to the dye bath after the dyed yarn had been removed. A reduction reaction proceeded at 60°C for 30 min.The dyed yarn wool was returned to the chromed dye bath, the temperature was increased to 95°C, and the wool was treated for 25 min (Figure 4).
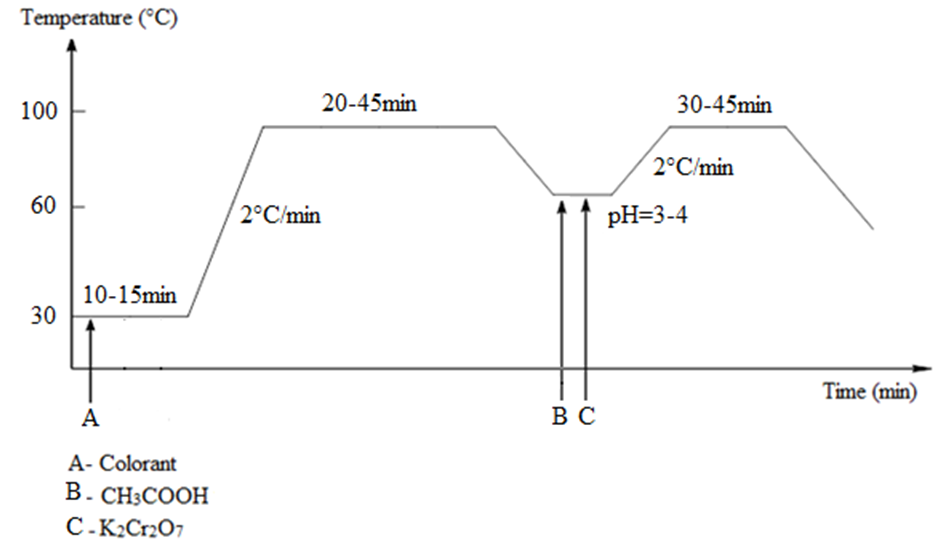 | Figure 4. Dyeing cycle of madder colorants and luteolin colorant |
2.3.7. Hot and Cold Rinse
- Rinsing of the samples (dyed with madder colorants and luteolin dye) was conducted at the end of the dyeing process to remove dyes from the fiber and inter-surfaces and to neutralize the acidic medium. Hot rinsing was carried out at 60°C for 15 min with a liquid ratio of 1/50. The cold rinse was conducted in a similar manner, except that it was undertaken at ambient temperature.
2.3.8. Drying
- Samples were dried in a sterile environment at 60°C and 80°C.
2.3.9. Washing Fastness
- The washing fastness was determined at 40°C over 30 min according to ISO 105-C6: A1S [24].
2.4. Surface Preparation of the Iron Metal
- Stripping of the iron sample was achieved using hydrochloric acid (HCl 50%), rinsing with distilled water, zincating in a 400-mL zinc bath by passing a direct current at 8 V for 15 min at room temperature, rinsing, passivating with a residual chromating dye bath (with a higher fixation rate of madder colorants and luteolin colorant) using nitric-acid addition (HNO3, 5%) at pH 1, and rinsing with hot water at 80°C.
3. Results and Discussion
3.1. Exhaustion Rate of Madder Colorants, Luteolin and Chrome in Dyes Bath
- The absorbance values of the initial and final dye bath are presented in Table 1.
|
3.2. Fixation Rate of Madder Colorants, Luteolin Colorant and Chrome in Dye Baths
- The absorbance values of the residual hot- and cold-rinse baths of the dyed samples are presented in Table 2.
|
3.3. Metal Surface Passivation with Residual Chromating Dye Bath
- The residual chromating dye bath was conserved for reuse during passivation of the iron surface. We selected the residual chromating dye bath that had achieved a higher fixation rate of madder colorants (61.8%). The passivation process described above led to a total absorption of chrome III on the metal surface, as demonstrated by the colour change from green to yellow in the passivation bath and on the metal surface. A complete transformation of hexavalent chrome VI to trivalent chrome III was achieved. Therefore, the residual chromating dye bath allowed the use of toxic chromium VI to be avoided.
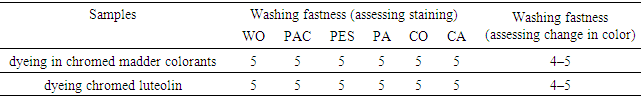
3.4. Washing Fastness
- Measurements for the two rinsed samples are presented in Table 3.
|
4. Conclusions
- This study confirmed the greater color fastness of the organometallic complex and its efficacy in the reuse of a residual chromating dye bath on metal surface passivation. Chromate-conversion coating technology has been used for decades, but hexavalent chrome is toxic and carcinogenic. Because the residual chromating dye bath was able to be reused for metal-passivation treatment, the resulting effluent was free from chrome and could be disposed of safely in the environment. Chromium salts act as an efficient mordant, promoting dye–metal complex formation to improve fastness, and they enhance acid–dye uptake because of its cationic nature when applied to wool before and after dyeing. However, the mordant affects the electron distribution and density within the dye, and the colour of the dyed fabric tends to change. This behaviour must be considered for shade establishment.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML