-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2018; 8(5): 99-106
doi:10.5923/j.chemistry.20180805.01

Effective and Fast Epoxidation Reaction of Linseed Oil Using 50 wt% Hydrogen Peroxyde
E. Dehonor-Márquez1, 2, J. F. Nieto-Alarcón1, 2, E. Vigueras-Santiago1, S. Hernández-Lopéz1
1Laboratorio de Desarrollo y Caracterización de Materiales Avanzados (LIDMA), Facultad de Química de Química de la Universidad Autónoma del Estado de México (UAEM). Paseo Tollocan Esquina con Paseo Colón, s/n. Moderna de la Cruz, Toluca, México
2Student in the Materials Science Program, UAEM
Correspondence to: S. Hernández-Lopéz, Laboratorio de Desarrollo y Caracterización de Materiales Avanzados (LIDMA), Facultad de Química de Química de la Universidad Autónoma del Estado de México (UAEM). Paseo Tollocan Esquina con Paseo Colón, s/n. Moderna de la Cruz, Toluca, México.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Epoxidation of linseed oil was performed with peracetic acid formed in situ by the reaction ofhydrogen peroxide and acetic acid in the presence of Amberlite 120H as catalyst, and toluene as solvent. Some variables were evaluated as temperature and the molar ratio and solution of aqueous hydrogen peroxide (30 and 50 wt%) for obtaining a maximum of yield and conversion of epoxidized oil. The characterization of conversion of double bonds (DB) to epoxy ring, the relative percentage of epoxidation and selectivity, were performed by 1H-NMR and FTIR-HART. The conditions rendered a maximum epoxidation of 93.4%, a conversion or double bonds of 97% and a selectivity of 96.3% were obtained at 80°C, employing a molar ratio 1:1.5 of double bonds (DB) to H2O2 (50 wt%), 1:0.5 of DB to acetic acid, and 25 wt% of catalyst, in only 50 min and with a good reproducibility (±1.1%).
Keywords: Epoxidation, Linseed oil, Acidic ion exchange resin
Cite this paper: E. Dehonor-Márquez, J. F. Nieto-Alarcón, E. Vigueras-Santiago, S. Hernández-Lopéz, Effective and Fast Epoxidation Reaction of Linseed Oil Using 50 wt% Hydrogen Peroxyde, American Journal of Chemistry, Vol. 8 No. 5, 2018, pp. 99-106. doi: 10.5923/j.chemistry.20180805.01.
Article Outline
1. Introduction
- Epoxides obtained from renewable sources [1-7] have application in a wide number of industrial and research processes: to obtain commercial products, and as reagents and intermediates used in the manufacture of polymers, resins, coatings, detergents, etc., generating a broad market and volume of consumption in the order of millions of tons per year. In this context, epoxidized vegetable oils (EVOs) [8-13] have been found viable due to their availability, low cost sustainability and non-toxicity; highlighting linseed oil, rapeseed oil and soybean oil for its higher production. The EVOs are commonly used as polyvinyl chloride (PVC) stabilizers, plasticizers [14], lubricants [15, 16], and starting materials to produce polyols [17-19], prepolymers [1, 20-24] and to synthesize polyurethane foams [25-29]. In addition, they are auxiliary agents used to improve the efficiency of linoleum production and the modification of other thermoset polymers [30-34]. The EVOs are usually produced at industrial scale with peroxocarboxylic acids formed in situ by reacting an organic acid as acetic acid, hydrogen peroxide and sulfuric acid [9, 35-41] (or any other soluble mineral acid) as the catalyst [42]. The search for optimize the epoxy yield conditions has been widely investigated [9, 42-50]: temperature, stirring speed, concentration of H2O2, type (homogeneous and heterogeneous) and amount of catalyst, acid precursor of the peroxyacid (formic, acetic, oleic), formation in situ of peroxyacid or addition of a preformed peroxocarboxylic acid, use or not of solvent, etc. Of particular interest is the epoxidation reaction using a heterogeneous catalyst as Lipase B [51-55], Alumina [56], Amorphous Ti/SiO2 [57-61], and Amberlite 120H. This last is an acidic ion exchange resin [62-71], which has been widely evaluated in many epoxidation reactions and has presented a better selectivity, less side reactions, it is easily recoverable and reusable, in comparison with the use of sulfuric acid as catalyst which are also more polluting and complex to separate, in addition to generating higher probability of secondary reactions, thereby reducing the epoxidation selectivity. The main results that gave the better conversion of DB to oxirane rings from the studies of epoxidation of several oils with Amberlite are [65] in the next ranges: temperatures oscillate between 60-75ºC, amount of catalyst range from 15 to 25%, high speed stirring (above 1500 rpm), a molar ration 1:0.5 of DB to acetic acid, a molar ratio 1:1.0-1:1.5 of DB to H2O2 (30%), with or without solvent (usually toluene or benzene) and a reaction time between 3 and 7 h. All those conditions also depending on the kind of oil, and the best parameter of the epoxidation reaction should be determined individually for each oil. Related with hydrogen hydroperoxide, there are a few researches that used H2O2 (50 wt%) mainly for safety hazard to the personnel and reactor equipment due the instability of the concentrate peroxides and peroxoacids [41, 44, 65]. Usually high temperatures are avoided when concentrate peracid is used. However, Haro [36] recently reported the epoxidation of grape seed oil at 90°C employing H2O2 (50 wt%), H2SO4 and acetic acid, rendered a conversion to epoxide of 90% in 60 min. At longer reaction times or higher temperatures than 90°C, aperture of epoxy groups and some oligomerization reactions usually have been detected [65]. In this work we evaluate the effect of the initial solution of hydrogen hydroperoxide, 30 and 50 wt% and the temperature at 65, 70 and 80°C, for epoxidation of linseed oil. Linseed oil is one of the most unsaturated triglycerides (6-6.4 DB) of particular interest for polymer synthesis. It would be very convenient to reach a conversion to epoxide up to 90% for this oil. As our better results, it was evidenced that using hydrogen peroxide at 50 wt% the conversion rate was fast reaching the maximal epoxidation (93.4%) in only 50 min at 80°C.
2. Methodology and Experimental
2.1. Reactants
- The solvents Toluene, Ethyl Acetate and Acetone; chromatographic-grade -Alumina, the catalyst Amberlite IR-120H (AIR-120H) and the reactive-grade Linseed Oil (LO) were supplied by Sigma-Aldrich, Co. LO consists of a clear yellow oil with a molecular weight of 865 g/mol, with a number of Iodine of 179.1 and 6.2 double bonds (DB) or ethylenic unsaturation, all determined by 1H-NMR according to [72-74]. Acetic Acid and the H2O2 (30 and 50 wt%) were purchased from Fermont. With exception of the LO, the other reagents were used as they were received. LO was passed through a packed column of -Alumina previous to use it, to eliminate the stabilizer.
2.2. Characterization
- FT-IR spectroscopic measurements were performed on a FT-IR Prestige 21, Shimadzu spectrometer, equipped with a horizontal attenuated total reflectance (HART) modus, with a crystal made of diamond. For quantitative analysis [17, 73], spectra were performed in Absorbance mode, a resolution of 4 cm-1, 64 scans in the range of 560 – 4000 cm-1. Spectra were normalized with respect to the area under the curve of the signal at 1745 cm-1 which corresponds to carbonyl vibration from ester groups of the triglyceride. The monitored signal by measure the area under the curve was the pair of bands centered at 821 and 798 cm-1 corresponding to the epoxy ring vibration.1H-NMR spectra were recorder using a Bruker Avance III a 300 MHz spectrometer, using CDCl3 as solvent and TMS as an internal standard. Quantification of conversion of DB, percentage of epoxidation and selectivity was calculated as described in [53, 72, 74].
2.3. Epoxidation Reaction Set up
- The evaluated variables were three temperatures: 65, 70 and 80ºC; the molar ratio of unsaturation respect to hydrogen peroxide (1:1 and 1:1.5) for the two aqueous solutions of hydrogen peroxide, 30 and 50%. Other values were fixed as the molar ratio of DB to acetic acid (1:0.5), a solvent quantity of 44 wt% and 25 wt% catalyst, both respect to the linseed oil weight. The times reactions were from 50 to 200 min and each reaction was made by triplicate. A general procedure consists in: place into a two-neck reactor equipped with a magnetic stirrer, thermometer and reflux condenser, 10 g of Linseed Oil (LO) equivalent to 72 mmol of DB, 2.1 g of Acetic Acid (AA), 4.4 g of Toluene (Tol) and 2.5 g of Amberlite IR-120H (AIR-120H). Reactor was placed in a water bath at 50°C for 15 min. The addition of 1:1 or 1:1.5 of H2O2 (30 or 50 wt%) started dropwise under vigorous stirring. Due the exothermic process, temperature is controlled to not to exceed the 50°C to avoid the decomposition of H2O2 [44, 67]. Once the addition of H2O2 was complete, the reaction mixture was heated up at the stablished temperature (65, 70 or 80°C). The reaction was monitored by FT-IR spectroscopy each 10-20 min (considering the initial time the completion of H2O2 addition), and when epoxidation was stopped to the determined time, the mixture was cooled to room temperature. It was filtered to recover the catalyst, which was washed with ethyl acetate to remove the excess oil and then with isopropyl ether and allowed to dry for reuse. The organic phase was washed several times with a saturated sodium bicarbonate solution until neutralization; the organic phase obtained was dried with anhydrous magnesium sulfate. After filtration the organic solvents (ethyl acetate and toluene) were evaporated using a rotary evaporator under reduced pressure. The product (ELO) was placed for 48 hours in a vacuum desiccator to eliminate traces of solvents for finally do the corresponding characterization by FT-IR-HART and 1H-NMR.
3. Results and Discussion
- Characterization of the epoxidized products was performed employing 1H-NMR and HART-FTIR spectroscopies. Usually the evaluation of the epoxidation reaction is employing analytical procedures; however, these processes involve time, chemical reactants, and they generate residues. High resolution NMR is an effective tool for determining iodine value of vegetable oils as determined by Miyaki, et al. [73]. He determined a correlation coefficient between traditional Wijs method and 1H NMR being it r2 = 0.9994 and within an error of ±1. Also, it is possible to calculate Iodine number and the molecular weight of the oil according to Natham and Díaz [72] and it has been a reliable technique for determine values of conversion, epoxidation and selectivity for an epoxidation reaction of oils [75]. FTIR spectroscopy is also an excellent tool for quantitative analysis. Using the relationship between the absorbance values and normalize the band of epoxide ring include the bands at 820 and 795 cm-1 it is possible to cancels out background, instrument noise, sample thickness, etc. Using an equation (1), it is possible monitoring the epoxidation of oils:
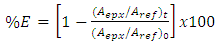 | (1) |
3.1. Characterization of LO
- FTIR-HART: Double bonds: 3009 cm-1 (=CH(ν), 1653.5 cm-1 (C=C(ν) and 719.5 cm-1 (C=C(cis-δ)). Methyl and methylene: 2956 cm-1 (terminal CH3(asym-νshoulder)), 2922.4 and 2853 cm-1 (-CH2(asym and sym-ν)) 1460 cm-1 (CH2(asym-δ)) and 1376.4 cm-1 (CH3(sym-δ). Ester carbonyl: 1743 cm-1 (C=O(ν)); 1159.2 cm-1 (C-O(ν)) and 719.5 cm-1 ((CH2)n(δ); being n ≥4). 1H NMR (300 MHz, CDCl3); δ in ppm (Integral, multiplicity, Hydrogen type):*0.88 (1.12, m, CH3- terminal groups); *0.97 (1.29, t, CH3- terminal group of linolenic acid); * 1.31 (10.13, d, -CH2-); *1.61 (1.55, s, -CH2-C=O); *2.05 (2.70, m, -CH2-CH2-CH=CH); *2.31 (1.51, t -CH2-C=O); *2.80 (1.82, m, -CH2-CH=CH-); *4.15 – 4.3 (1, dd, CH2-O of glyceride); *5.35 (3.37, m, -CH=CH- and CH-O of glyceride).
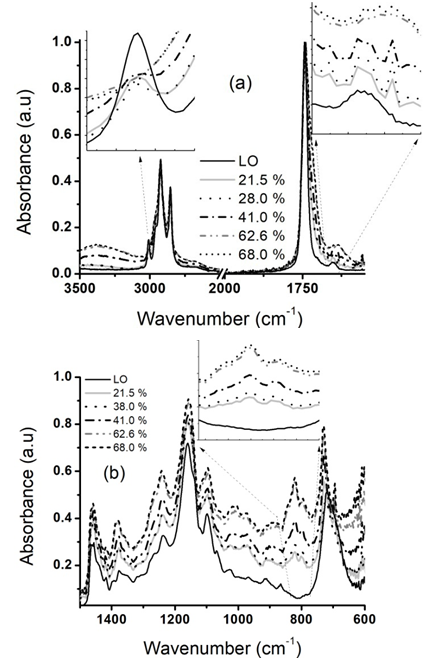
3.2. Following of the Epoxidation Reaction
- In Figure. 1a, could be appreciated how the FTIR-normalized signals of LO corresponding to double bonds vibrations in 3009 cm-1 (=CHstr) and 1653.5 cm-1 (C=C(ν)) vanished in the spectrum of the epoxidized product (insets from Figure. 1a), whereas the band at 719.5 cm-1 (C=CH(δ) plus (CH2)n(δ); being n ≥4) only diminishes in intensity as the reaction performed at 80°C, a molar ratio 1:1 LO to H2O2 (30 wt%) proceeds (run 9, Table 1). However, the two bands of the epoxy ring vibration (inset from Figure. 1b) centered between 820 and 795 cm-1 were observed in the first minutes of reactions and they increase as the reaction take place. This signal is the one is quantified with the course of the reaction until the stablished reaction time [17]. Both spectra (LO and ELO) have signals that match (do not change) with the course of the reaction. This bands corresponding to methyl and methylene groups at 2956, 2922.4, 2853, 1460 and 1376.4 cm-1 as well as the vibration corresponding to the ester carbonyl at 1743.6 and 1159.2 cm-1.
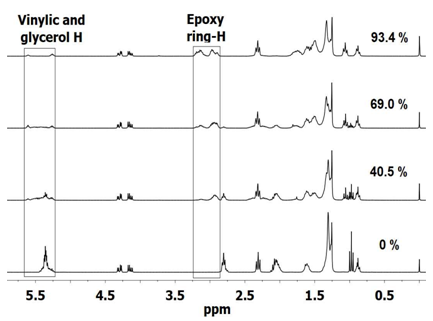 | Figure 2. 1H NMR spectra for different conversion of epoxide: 0% for LO, 40.5% (run 2, Table 1), 69.0% (run 3, Table 1) and 93.4% (run 3, Table 2) |
3.3. Effect of the Concentration of the Solution of Hydrogen Peroxide: 30 and 50 wt%, and Temperature
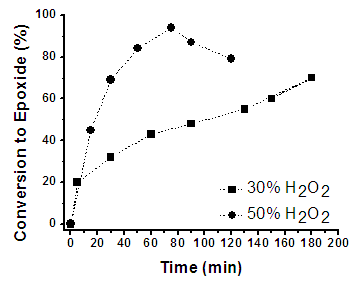
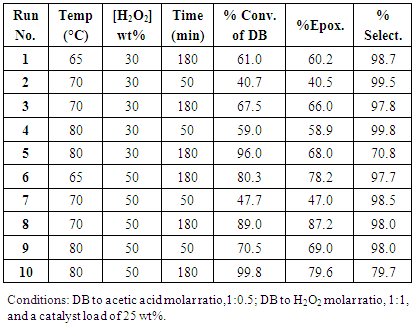
- Epoxidation of Linseed oil with peracetic acid formed in situ in the presence of an ion-exchange resin in a heterogeneous catalytic process. Peracetic acid diffuses through the water to the oil phase for reacting with the unsaturation of the oil to forming an epoxy group [42, 65]. This is the description of an ideal reaction however, has been well studied that excess of some reactants and/or changes on parameters as temperature, usually render side undesired reactions [42, 65, 67].Figure 3 shows the sharp increase of the epoxidation conversion when H2O2 (50 wt%) is employed in comparison with H2O2 (30 wt%) at 80°C. The curve for H2O2 (30 wt%) was built with the data obtained from Figure 1. For reaction using H2O2 (50 wt%) the maximal conversion (93%) was achieved in 75 min, after this time the amount of epoxy groups started to diminish due side aperture reactions; whereas for reaction ran with H2O2 (30%) at the same time (75 min) it was only reached a 40% of conversion. This tangible difference between using H2O2 at 30 or 50 wt% is due to the higher concentration of active oxygen in H2O2 (50 wt%) that increases the rate of in situ formation of peracetic acid, and as consequence the rate of epoxidation of the DB in LO. Analyzing the runs 1 and 6 in Table 1, which were performed at 65°C but using H2O2 at 30 and 50 wt% respectively, it is notice an increase of 23% in the epoxidation percent at 180 min. For runs 2 and 7 carried out at 70°C, there was an increment on the conversion to epoxide of 37% in 50 min, while for reactions performed at 80°C (runs 4 and 9) there was only an increment of 15% in 50 min; however at this time the epoxidation conversion has reached 70%.
|
3.4. Effect of the Molar Ratio of DB to H2O2 (50 wt%) and Temperature
- It was demonstrated first the effect on increasing the molar ratio of DB to H2O2 (50 wt%) from 1.0:1.0 to 1.0:1.5 at 80°C. Figure. 4 shows the that the molar ratio 1:1.5 of DB to H2O2 (50 wt%) was the most advantageous for epoxidation of linseed oil; there was a noticeable increasing on the rate of epoxidation at 80°C. The maximal conversion for both molar ratio is practically the same (93-94) however it takes 75 min when a molar ratio 1.0:1.0 es employed and only 50 min when a molar ratio 1.0:1.5 is added. After those times, the conversion started to diminish in both cases. The use of H2O2 (50%) and a high molar ratio as well 80°C of temperature; the reaction started to take place very fast and favorable in the first 30 min. At this time almost 80% of epoxides has been formed whereas only 60% of them are quantified when a molar ratio 1.0:1.0 has been employed.
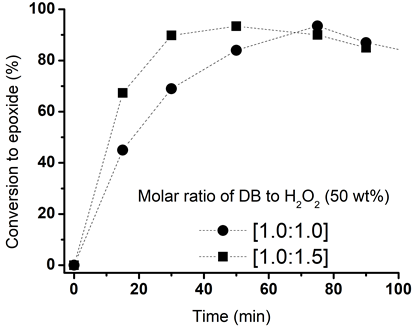 | Figure 4. Effect of increasing the molar ratio of DB to H2O2 (50 wt%) on the conversion to epoxide. Other conditions: DB to acetic acid molar ratio, 1:0.5; and 25 wt% of catalyst, 80°C |
|
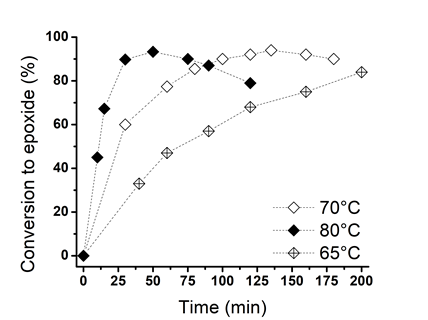 | Figure 5. Effect of the temperature on the conversion to epoxide. Other conditions: DB to acetic acid molar ratio, 1:0.5; DB to H2O2 (50%), 1:1 molar ratio, and 25 wt% of catalyst |
4. Conclusions
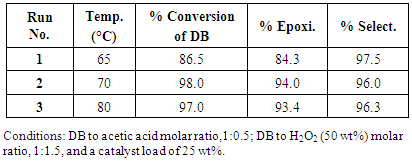
- The results of the present investigation show that Linseed Oil could be efficiently epoxidized in a short time. The combination of parameters that render the better results of the epoxidation reaction of LO are: a molar relation of 1:0.5:1.5 of DB to acetic acid and H2O2 (50 wt%), 25 wt% of catalyst, 80°C and 50 min. As well the conditions: a molar relation rate of 1:0.5:1.5 of DB to acetic acid and H2O2 (50 wt%), 25 wt% of catalyst, 70°C and 135 min, rendered practically the same results: conversion of DB, 97-98%; conversion to epoxide, 93% (± 1%) and a selectivity of 96%. The monitoring and characterization of the reaction was performed by FTIR-HART and 1H NMR spectroscopies. The double bonds and epoxy signals were well identified and quantified employing both spectroscopies, evidenced the good reliability of them for characterize the epoxidized oils.
ACKNOWLEDGEMENTS
- Authors thank to the CONACyT for the fellowship and to the M.C. Nieves Zavala from CCIQS, UAEM-UNAM for her technical support for the NMR analysis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML