-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2018; 8(4): 96-98
doi:10.5923/j.chemistry.20180804.03

MgBr.OEt2 Promoted Synthesis of Functionalized Carbocyclics from Simple Precursors
Bello Y. Makama, Wahidullah Azizi
Division of Mathematics, Sciences & Technology, American University of Afghanistan, Darul-Amman, Kabul, Afghanistan
Correspondence to: Bello Y. Makama, Division of Mathematics, Sciences & Technology, American University of Afghanistan, Darul-Amman, Kabul, Afghanistan.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Magnesium bromide diethyl etherate has been employed to promote the ene-reaction of α,β-unsaturated lactones. The cylicizations described herein provide a very general approach to the synthesis of a well-functionalized bicycles from simple acyclic precursors mediated by magnesium bromide diethyl etherate and other Lewis acids.
Keywords: Magnesium bromide diethyl etherate, the ene-reaction, α,β-unsaturated lactones, Lactones, Lewis acids
Cite this paper: Bello Y. Makama, Wahidullah Azizi, MgBr.OEt2 Promoted Synthesis of Functionalized Carbocyclics from Simple Precursors, American Journal of Chemistry, Vol. 8 No. 4, 2018, pp. 96-98. doi: 10.5923/j.chemistry.20180804.03.
1. Introduction
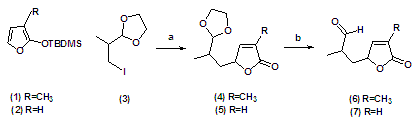 (a) silver trifluoroacetate, DCM, 55% (b) TsOH, acetone/water, reflux, 43%We have reported our success with the Lewis acids catalysed ene-reaction of 5-(But-3-enyl)furan-2(5H)-one. [1] The success encountered in attempting to effect the cyclization via ene-reaction led us to focus on the cyclization of 2-methyl-3-(4-methyl-5-oxo-2,5-dihydrofuran-2-yl) propanal (6) and relatad precursor (7) as the key synthetic objective of this report. The simple transformation necessary to convert (6) and (7) was anticipated to proceed through MgBr.OEt2 catalysed ene-reaction. We have also previously reported the condensation of 2-(iodopropan-2-yl)-1,3-dioxolane (3) and 2-(tert-butyldimethylsiloxy)-3-methylfuranone (1) in DCM and silver trifluoroacetate to furnish 5-(2-(1,3-dioxalan-2yl)propyl)-3-methylfuran-2-(5H)-one (4) as a pair of diastreoisomers with an overall yield of 55%. [2, 3, 4] In the same vein we reported the synthesis of the desired aldehyde under mild acid conditions [5, 6], and was achieved via reaction with PTSA in acetone/water (5:1), this being the most conventional and straightforward method for the removal of the dioxolane. Herein we are reporting our preliminary findings with magnesium bromide diethyl etherate promoted cyclization of and (6) and (7). We have also attempted to demonstrate the efficacy of other Lewis acids in the cyclization protocol.
(a) silver trifluoroacetate, DCM, 55% (b) TsOH, acetone/water, reflux, 43%We have reported our success with the Lewis acids catalysed ene-reaction of 5-(But-3-enyl)furan-2(5H)-one. [1] The success encountered in attempting to effect the cyclization via ene-reaction led us to focus on the cyclization of 2-methyl-3-(4-methyl-5-oxo-2,5-dihydrofuran-2-yl) propanal (6) and relatad precursor (7) as the key synthetic objective of this report. The simple transformation necessary to convert (6) and (7) was anticipated to proceed through MgBr.OEt2 catalysed ene-reaction. We have also previously reported the condensation of 2-(iodopropan-2-yl)-1,3-dioxolane (3) and 2-(tert-butyldimethylsiloxy)-3-methylfuranone (1) in DCM and silver trifluoroacetate to furnish 5-(2-(1,3-dioxalan-2yl)propyl)-3-methylfuran-2-(5H)-one (4) as a pair of diastreoisomers with an overall yield of 55%. [2, 3, 4] In the same vein we reported the synthesis of the desired aldehyde under mild acid conditions [5, 6], and was achieved via reaction with PTSA in acetone/water (5:1), this being the most conventional and straightforward method for the removal of the dioxolane. Herein we are reporting our preliminary findings with magnesium bromide diethyl etherate promoted cyclization of and (6) and (7). We have also attempted to demonstrate the efficacy of other Lewis acids in the cyclization protocol.2. Results & Discussion
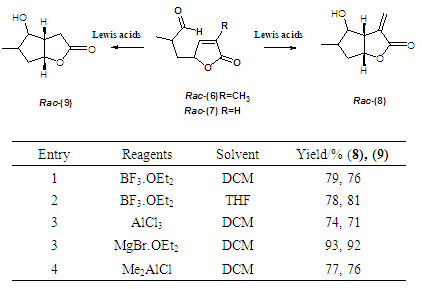
- MgBr.OEt2 promoted ene reaction of key intermediates (6) and (7) The attempted synthesis of the bicycles system (8) and (9) was to involve the reactions of (6) and (7) using an ene-reaction as reported by Snider. [7, 8, 9] According to this method, MgBr.OEt2 was added to solutions of (6) and (7) each in DCM at -78°C under nitrogen and the resulting mixtures were allowed to warm to room temperature with stirring for 48 hours. After workup, 1H NMR analysis revealed (8) and (9) had been formed in an overall yield of 93%. The reaction was repeated with different Lewis acids to isolate the rac- (8) and rac- (9) in good yields. The results from the Lewis Acids catalyzed ene-reaction of the two precursors is shown in table 1. The stereochemical assignment of -cis at ring junction was achieved via nOe.
3. Material and Methods
- Commercial reagents were obtained from Aldrich and Lancaster chemical suppliers and were used directly as supplied or purified prior to use following the guidelines of Perrin and Amarego. [10] Dichloromethane and acetonitrile were refluxed over and distilled from CaH2 prior to use. Light petroleum is the fraction of petroleum ether boiling in the range 30-40°C, and it was fractionally distilled through a 36 cm Vigreux column before use. Non-aqueous reagents were transferred under argon via syringe. Organic solutions were concentrated under reduced pressure on a Büchi rotary evaporator using a water bath. Thin-layer chromatography (TLC) was performed on Merck aluminium-backed plates coated with 0.2 mm silica gel 60-F plates. Visualization of the developed chromatogram was performed by UV fluorescence quenching at 254nm, or by staining with a KMnO4 solution. 1H and 13C NMR spectra were recorded on a Bruker DPX250 (250 MHz for protons) and a Brüker AMX400 (400 MHz for protons). Data for 1H NMR are reported as follows: chemical shift (δ-ppm), multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet), integration, coupling constant in (Hz). Data for 13C NMR spectra are reported in terms of chemical shift (ppm) down field from TMS. IR spectra were recorded on a Perkin Elmer Paragon 1000 or a Perkin Elmer 881 spectrometer as a thin film between sodium chloride plates or as a KBr disk. All absorptions are reported in terms of frequency of absorption (cm-1). Mass spectrometric data were recorded on VG Autospec, under conditions of chemical ionisation (C.I) using ammonia as the ionising source. Peaks are quoted in the form (m/z) (relative intensity). Rac- (8)
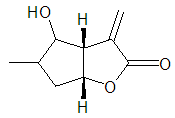 To a stirred a solution of 2-methyl-3-(4-methyl-5-oxo-2,5-dihydrofuran-2-yl) propanal (6) (200 mg, 1.19 mmol, 1 equiv) in dry dichloromethane (10 mL) under argon -78°C was added MgBr.OEt2 (387 mg, 1.5 mmol, 1.3 equiv) and reaction was stirred for 24 h by which time TLC analysis revealed the formation of a new product. Water (12 mL) was added and the organic layer separated, the residue was extracted with DCM (4 x 10 mL). The DCM layer was washed with cold aqueous saturated sodium hydrogen carbonate (2 x 5 mL). The solvents were removed in vacuo and the residue was purified by flash column chromatography on silica, eluting with hexane : ethyl acetate (1:1) to furnish (8) as a colourless oil (186 mg, 93%); υmax (thin film/cm-1), 3221, 2930, 2857, 17541, 1644, 1088, 922; δH (400 MHz, CDCl3) 6.18 (1H, d, CH2=C), 5.91 (1H, d, CH2CH), 4.11 (1H, q, J 5.4 Hz, J 2.0 Hz, CHO), 3.41 (1H, triplet, CHOH, J 5.3 Hz), 2.83 (1H, dd, J 5.4 Hz, J 2.1 Hz, CHCHO), 1.94-1.78 (1H, m, CHCH3), 1.78-1.1.56 (2H, m, CH2CHCH3), 1.09 (3H, d, J 6.5 Hz, CH3); δC (100 MHz, CDCl3) 171.3, 140.1, 120.3, 86.4, 73.1, 39.5, 32.5, 31.7, 15.7; m/z (C.I) 153 (MH+, 100%), C9H12O3, requires 169.0866, found 169.085; Rac-9
To a stirred a solution of 2-methyl-3-(4-methyl-5-oxo-2,5-dihydrofuran-2-yl) propanal (6) (200 mg, 1.19 mmol, 1 equiv) in dry dichloromethane (10 mL) under argon -78°C was added MgBr.OEt2 (387 mg, 1.5 mmol, 1.3 equiv) and reaction was stirred for 24 h by which time TLC analysis revealed the formation of a new product. Water (12 mL) was added and the organic layer separated, the residue was extracted with DCM (4 x 10 mL). The DCM layer was washed with cold aqueous saturated sodium hydrogen carbonate (2 x 5 mL). The solvents were removed in vacuo and the residue was purified by flash column chromatography on silica, eluting with hexane : ethyl acetate (1:1) to furnish (8) as a colourless oil (186 mg, 93%); υmax (thin film/cm-1), 3221, 2930, 2857, 17541, 1644, 1088, 922; δH (400 MHz, CDCl3) 6.18 (1H, d, CH2=C), 5.91 (1H, d, CH2CH), 4.11 (1H, q, J 5.4 Hz, J 2.0 Hz, CHO), 3.41 (1H, triplet, CHOH, J 5.3 Hz), 2.83 (1H, dd, J 5.4 Hz, J 2.1 Hz, CHCHO), 1.94-1.78 (1H, m, CHCH3), 1.78-1.1.56 (2H, m, CH2CHCH3), 1.09 (3H, d, J 6.5 Hz, CH3); δC (100 MHz, CDCl3) 171.3, 140.1, 120.3, 86.4, 73.1, 39.5, 32.5, 31.7, 15.7; m/z (C.I) 153 (MH+, 100%), C9H12O3, requires 169.0866, found 169.085; Rac-9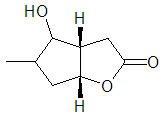 To a stirred a solution of 2-methyl-3-(5-oxo-2,5-dihydrofuran-2-yl) propanal (7) (100 mg, 0.65 mmol, 1 equiv) in dry dichloromethane (7 mL) under argon -78°C was added MgBr.OEt2 (219 mg, 0.85 mmol, 1.3 equiv) and reaction was stirred for 48 h by which time TLC analysis revealed the formation of a new product. Water (12 mL) was added and the organic layer separated, the residue was extracted with DCM (4 x 10 mL). The DCM layer was washed with cold aqueous saturated sodium hydrogen carbonate (2 x 5 mL). The solvents were removed in vacuo and the residue was purified by flash column chromatography on silica, eluting with hexane: ethyl acetate (1:1) to furnish Rac-9 as a colourless oil (93 mg, 92%); υmax (thin film/cm-1), 3649, 3566, 2857, 1741, 1634, 1011; δH (400 MHz, CDCl3) 4.21 (1H, q, J 5.4 Hz, J 2.0 Hz, CHO), 3.71 (1H, triplet, J 5.3 Hz, CHOH), 2.03-1.99 (1H, m, CHCHO), 1.94-1.78 (1H, m, CHCHO), 1.79-1.1.54 (3H, m, CH2CHCH3, CHCH3), 1.04 (3H, d, J 6.5 Hz, CH3); δC (100 MHz, CDCl3) 174.1, 90.4, 80.4, 38.1, 33.5, 31.5, 29.7, 29.5, m/z (C.I) 153 (MH+, 100%), C8H12O3, requires 157.087, found 157.088.
To a stirred a solution of 2-methyl-3-(5-oxo-2,5-dihydrofuran-2-yl) propanal (7) (100 mg, 0.65 mmol, 1 equiv) in dry dichloromethane (7 mL) under argon -78°C was added MgBr.OEt2 (219 mg, 0.85 mmol, 1.3 equiv) and reaction was stirred for 48 h by which time TLC analysis revealed the formation of a new product. Water (12 mL) was added and the organic layer separated, the residue was extracted with DCM (4 x 10 mL). The DCM layer was washed with cold aqueous saturated sodium hydrogen carbonate (2 x 5 mL). The solvents were removed in vacuo and the residue was purified by flash column chromatography on silica, eluting with hexane: ethyl acetate (1:1) to furnish Rac-9 as a colourless oil (93 mg, 92%); υmax (thin film/cm-1), 3649, 3566, 2857, 1741, 1634, 1011; δH (400 MHz, CDCl3) 4.21 (1H, q, J 5.4 Hz, J 2.0 Hz, CHO), 3.71 (1H, triplet, J 5.3 Hz, CHOH), 2.03-1.99 (1H, m, CHCHO), 1.94-1.78 (1H, m, CHCHO), 1.79-1.1.54 (3H, m, CH2CHCH3, CHCH3), 1.04 (3H, d, J 6.5 Hz, CH3); δC (100 MHz, CDCl3) 174.1, 90.4, 80.4, 38.1, 33.5, 31.5, 29.7, 29.5, m/z (C.I) 153 (MH+, 100%), C8H12O3, requires 157.087, found 157.088. Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML