-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2018; 8(4): 85-89
doi:10.5923/j.chemistry.20180804.01

On the Mechanism of Indirubin Formation in the Baeyer-Emmerling Synthesis
Francisco Sánchez-Viesca, Reina Gómez
Organic Chemistry Department, Faculty of Chemistry, National Autonomous University of Mexico, Mexico City (CDMX), México
Correspondence to: Francisco Sánchez-Viesca, Organic Chemistry Department, Faculty of Chemistry, National Autonomous University of Mexico, Mexico City (CDMX), México.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Indigo red, the co-product formed in the Baeyer-Emmerling indigo synthesis, has been synthesized by two different methods. One is from Baeyer and the other is due to Wahl. The last procedure has been improved afterwards. However, both methods employ reactants that are absent in the Baeyer-Emmerling synthesis. Thus, the way by which indirubin (indigo red) is formed in the reaction medium of the original synthesis is unknown. We have cleared this and also why this co-product is always present in this synthesis. Baeyer intended a regioselective synthesis of indigo blue, but many years later biological activity was found in the co-product and now this important compound is used in cancer treatment.
Keywords: Indigo blue, Indigo red, Indirubin, Purpurin, Reaction mechanisms, Reactive intermediates
Cite this paper: Francisco Sánchez-Viesca, Reina Gómez, On the Mechanism of Indirubin Formation in the Baeyer-Emmerling Synthesis, American Journal of Chemistry, Vol. 8 No. 4, 2018, pp. 85-89. doi: 10.5923/j.chemistry.20180804.01.
1. Introduction
- The first synthesis of indigo was accomplished by Baeyer and Emmerling, and Baeyer improved it for ten years. It has been updated by us in a critical review [1].The present communication is devoted to the co-product that is formed in this synthesis. An unexpected fact was the presence of another coloured product (indigo red) which first was termed indigo purpurin and years later Baeyer named it indirubin. This co-product troubled Baeyer since he desired obtain indigo blue as the sole product.Natural indigo, isolated from different plants, also contains the red isomer. The beneficial properties of indigo were discovered by Chinese Medicine, and afterwards it was cleared that the red component was the actual responsible for the medicinal properties. Actually indirubin is used in cancer treatment [2, 3].There are two syntheses of indirubin, one is from Baeyer and the other is from Wahl. However, in both syntheses are used alien reactants that are not present in the Baeyer-Emmerling procedure.Now we present two routes showing how indirubin is formed in the Baeyer-Emmerling synthesis, and in the subsequent modifications of it. The provided reaction mechanisms not only explain the formation of indirubin, but also why indigo red is always produced as a companion of indigo blue in this synthesis, and in a minor proportion.
2. Antecedents
- Baeyer and Emmerling achieved the first synthesis of indigo [4, 5]. This is remarkable because at that time the structure of indigo was unknown. They knew that treatment of natural indigo with nitric acid gave an oxidation product, isatin, whose chemical structure had been advanced by Kekulé just one year before and was not yet well established.Baeyer attempted the reverse reaction, isatin reduction. However, other products than indigo were obtained, depending on the employed reagents and the raction conditions. So, Baeyer and Emmerling treated isatin with phosphorus trichloride with the purpose of increasing the reactivity of the resulting chloro derivative. The reaction is based on the lactam-lactim isomerism, the phosphorus halide acting on the imidol. We provide the reaction mechanism in Figure 1.
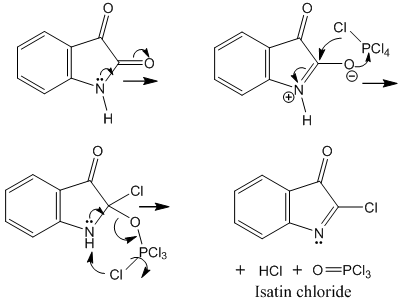 | Figure 1. Preparation of isatin chloride from isatin and phosphorus pentachloride via the amphion |
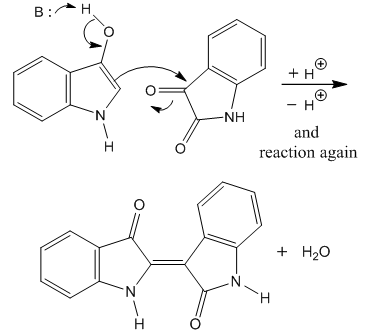 | Figure 2. Baeyer’s indirubin (indigo purpurin) synthesis |
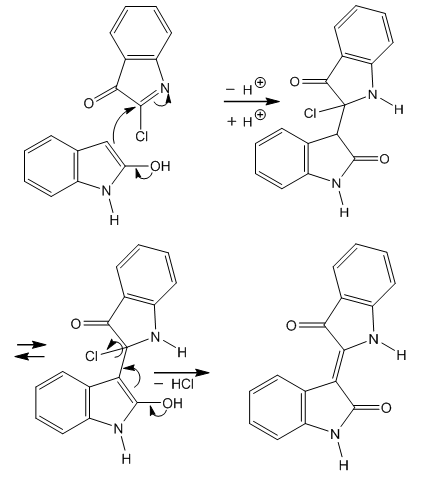 | Figure 3. Wahl’s indirubin synthesis from oxindole as 2-hydroxyindole |
3. Discussion
- Now let’s see if indoxyl or oxindol used in the previous syntheses can be formed in the Baeyer-Emmerling reaction medium.Indoxyl (3-hydroxyindole or indolin-3-one) is discarded since it would come from partial reduction of isatin, the starting reactant. However the more reactive group in isatin is the ketone, not the lactam. For instance, Baeyer reduced isatin with zinc and dilute hydrochloric acid and obtained dioxindole (3-hydroxyoxindole), which he named hydroisatin and gave it the formula of enediol [15, 16], Figure 4.
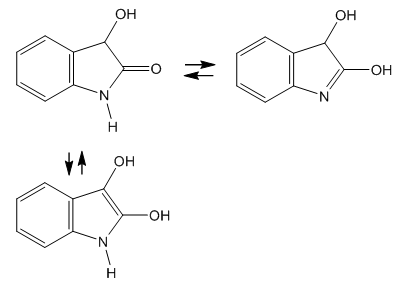 | Figure 4. Isomeric structures of dioxindole |
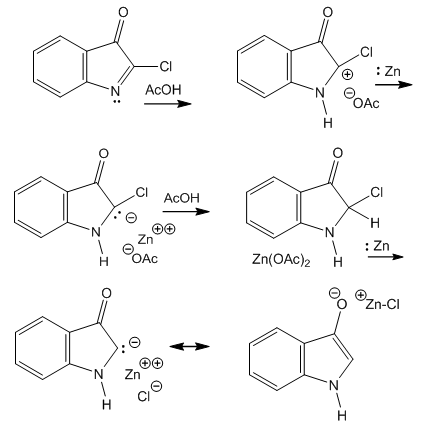 | Figure 5. Three-reactant reaction medium from which the products derive, showing chloro zinc enolate with C- or O- metallation |
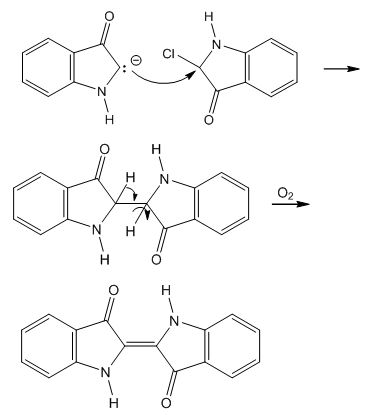 | Figure 6. Obtention of indigo blue from the intermediate α-chloro ketone |
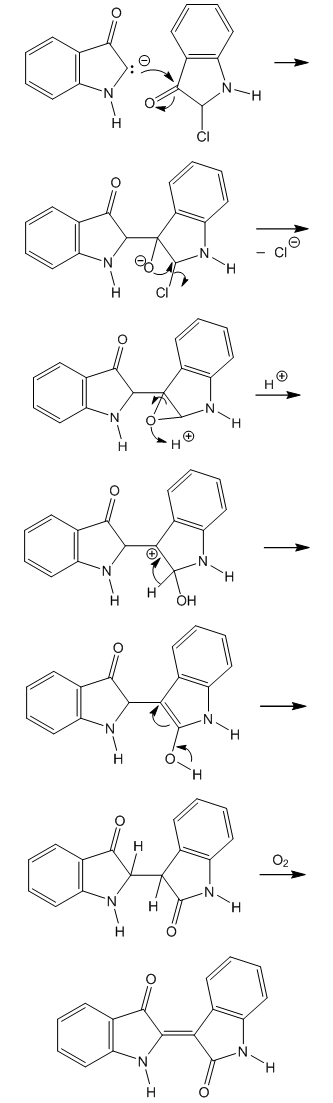 | Figure 7. Synthesis of indirubin from the intermediate α-chloro ketone |
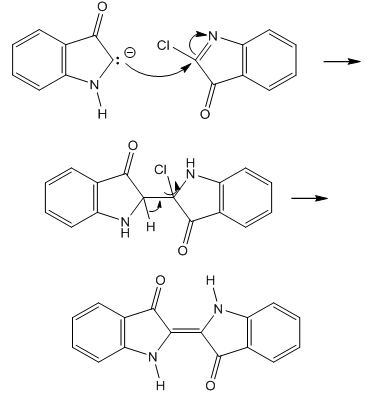 | Figure 8. Indigo blue formation from isatin chloride |
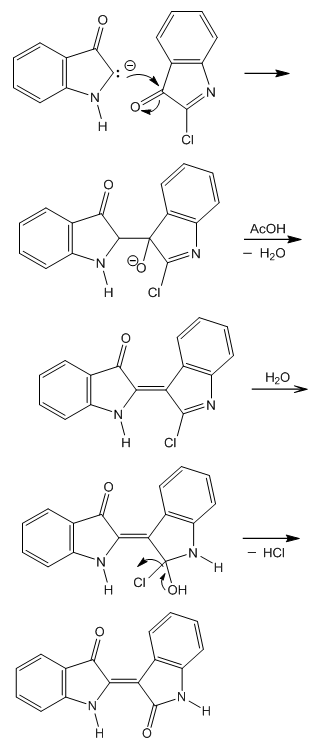 | Figure 9. Indirubin synthesis from isatin chloride |
4. Conclusions
- It is a challenge for the theorist to unravel the course of a reaction, providing a sound reaction mechanism that is in accordance with the experimental facts and known chemical deportment.We have found four routes to explain indirubin and indigo formation, two for each isomer.There is a three-component reaction medium from which arise the obtained products. The nucleophile is a chloro zinc enolate and there are two electrophiles, isatin chloride (2-chloroindolenin-3-one), and 2-chloroindolin-3-one.Each electrophile has two reactive sites. Indigo blue, obtained in higher yield, derives from reaction at the more reactive electrophilic sites, whereas indirubin (indigo red) comes from reaction at the less reactive site in each electrophile. By means of these intertwined reactions we have correlated yields with regiochemistry.The presented reaction mechanisms not only explain how the products are formed but also why indirubin is always present as co-product in the Baeyer-Emmerling synthesis, and in a lesser proportion than indigo blue.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML