-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2018; 8(3): 65-71
doi:10.5923/j.chemistry.20180803.02

Preparation of Sulphide-lime Unhairing Ratios for Pollution Load Reduction in the Manufacture of Shoe Upper Leathers from Tropical Goat Skins
Tanko S. F.1, Ajibola V. O.2, Agbaji E. B.2, Idris S. O.1, 2, Putshaka J. D.1, Amana B. S.1
1Institute of Leather and Science Technology, Zaria, Nigeria
2Department of Chemistry, Ahmadu Bello University, Zaria, Nigeria
Correspondence to: Tanko S. F., Institute of Leather and Science Technology, Zaria, Nigeria.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Preparation of sulphide-lime unhairing tannery process was carried out using nine ratios coded as AI – AIII; BI - BIII and CI – CIII with varied concentrations of sodium sulphide and hydrated lime. Result obtained through subjective tests and gravimetric method show differences in degree of unhairing, swelling and plumping of the pelts. The pH of wastewater generated from each ratio and weight increase of limed pelts, was observed and recorded. Analysis of pollutants from the wastewater was determined by parameters such as BOD, COD, and Sulphide generated from the wastewater. Results obtained show various levels of parameters from the studied ratios indicated that A-group had poor unhairing and liming properties. The C-group gave excellent properties but with higher pollution load. However, BII (2.5% Na2S with 2.0% Ca(OH)2) and BIII (2.8% Na2S with 2.5% Ca(OH)2) in 200% float generated lower pollution level as determined by the parameters from their individual wastewaters. This was compared with the conventional (CTL) and that of the industry. The average sample wastewater collected from the industry indicated higher pollutants than the studied and CTL ratios. The study reveals that maximum utilization of reagents is a necessary technique for reduction of pollutants in the wastewater from sulphide-lime unhairing process known to generate 65%-70% of pollutants from the beam-house.
Keywords: Preparation, Unhairing, Liming, Ratios, Wastewater
Cite this paper: Tanko S. F., Ajibola V. O., Agbaji E. B., Idris S. O., Putshaka J. D., Amana B. S., Preparation of Sulphide-lime Unhairing Ratios for Pollution Load Reduction in the Manufacture of Shoe Upper Leathers from Tropical Goat Skins, American Journal of Chemistry, Vol. 8 No. 3, 2018, pp. 65-71. doi: 10.5923/j.chemistry.20180803.02.
Article Outline
1. Introduction
- Unhairing/liming is a mandatory process especially in the manufacture of full grain leathers from goat skins. The process removes epidermal structures, brings about swelling and plumping and the removal of non-structural protein of the pelts [1]. Liming process is the stage, that properties of leathers are largely determined thus “leather is made or marred during liming” [2]. The process modifies the skin fibre for subsequent penetration of other processing chemicals such as tannins which has direct bearing with both physical and chemical properties of the resultant leathers [3].Sodium sulphide and hydrated lime combination is popularly used in this process because other methods of unhairing and liming have one setback or the other [4, 5]. However, the use of this chemical combination generates large volumes of waste that constitutes major source of pollutants amounting to more than 65-70% in combined tannery effluent [6, 7]; Leaf, 1999. The effluent which constitutes both organic, inorganic matter and metals makes its treatment very complicated and expensive [8, 9]. If this wastewater is not properly treated before discharge may affects the ecology of agricultural land and other factors of the environment as reported by some researchers [10].It is therefore important to prepare a sodium sulphide-hydrated lime combination ratio that will results to minimal pollutants in the unhairing-liming wastewater and consequently to the overall tannery effluent [11, 12]. Pollution load associated with tannery wastewater, has been reported as the greatest challenge in the leather industry [13, 14]. Preparation of chemical combination of sulphide-lime in unhairing-liming process is necessary for the reduction of pollutants in its effluent [15, 16].
2. Materials and Methods
- Wet-Salted medium Goatskins were obtained from Tudun-wada, Zaria, Nigeria. They were soaked, washed free from dirt and other extraneous substances and drained. Each piece of skin was divided into two halves along the back-bone and weighed prior to each batch of unhairing-liming ratio. The sulphide-lime unhairing process was carried out according to standard procedures. Three (3) classes of ratios were drawn for this study and coded as;Class ‘A’ RatiosClass ‘A’ ratios used 2.5% w/w sodium sulphide for unhairing-liming process. The quantity of calcium hydroxide was varied accordingly, 1.5%, 2.0%, 2.5% and 3.0% in a float of 200% w/v for the water (all percentages based on weight of each skin). Four ratios were formulated with the fifth as conventional ratio serving as control (CTL).Ratio AI: - 2.5 % Na2S/1.5 % Ca (OH)2,Ratio AII: -2.5 % Na2S/2.0% Ca OH)2,Ratio AIII: -2.5 % Na2S/2.5 % Ca (OH)2,Ratio AIV: -2.5 % Na2S/3.0 % Ca (OH)2CTL - 3.0 % Na2S/3.0 % Ca(OH)2E - Wastewater from commercial Tanneries.For Ratio AI, 21.25g of sodium sulphide was dissolved in 1000 mls water and transferred quantitatively into an experimental drum (reactor). The soaked skins weighing 850 g were introduced into the vessel and agitated for 3 hours continuously at a speed of 60 rpm until the skins were unhaired. Then 12.75g of hydrated lime was dissolved in 200 ml of water and transferred into the vessel and agitated for another 2 hours. Then 500 ml of water was added and agitated for 5 minutes every one hour for the remaining 15 hours. The process was repeated for AII but the percentage of lime was increased to 2.0% according to the ratio. The procedure was repeated for AIII and AIV in class ‘A’. After unhairing-liming of the skins, the degree of unhairing, swelling, plumping was assessed for each skin and recorded. Samples of wastewater from each ratio and the CTL were collected after the process period of 20 hours. The ratios coded as AI – AIV and wastewater from each was collected and refrigerated at 4°C until required for analysis. Parameters such as COD, BOD and S2- were used for the determination of pollution load of the wastewater. Samples of wastewater from three commercial tanneries from Kano-Nigeria were also analysed for the same parameters.Class ‘B’ RatiosIn this class, sample skins weighed 880g; the same unhairing-liming method was adopted like in class ‘A’ except for the increase in percentage of sodium sulphide to 2.8%.Ratio BI: - 2.8 % Na2S/1.5 % Ca(OH)2Ratio BII: - 2.8 % Na2S/2.0 % Ca(OH)2,Ratio BIII: - 2.8 % Na2S/2.5 % Ca(OH)2,Ratio BIV:- 2.8 % Na2S/3.0 % Ca(OH)2,CTL: - 3.0 % Na2S/3.0 % Ca(OH)2,E – Wastewater from commercial Tanneries.For BI 24.64g of sodium sulphide was dissolved in 1000 ml of water and transferred quantitatively to the reactor containing the skins and agitated for 3 hours until they were unhaired. Then 13.2g of lime was dissolved in 300 ml of water and added into the same drum and agitated for another 2 hours continuously. Finally 460 ml of water was added and agitated for 15 hours at 5 minutes every one hour. The procedure was then repeated for BII, BIII and BIV. The same variation in the percentage of hydrated lime was used; 1.5%. 2.0% 2.5% and 3.0%. The limed pelts were assessed subjectively as previously carried out in class A. Samples wastewater from the individual ratio coded as (BI – BIV) were collected after unhairing-liming period of 20 hours and analysed for the same parameters like in class ‘A’.Class C RatiosFor class ‘C’ the same procedure was repeated like in class ‘B’ except for the increase in percentage of sodium sulphide to 3.0% w/w. The percentage of hydrated lime remained as 1.5%, 2.0%, 2.5% and 3.0%.Ratio CI: – 3.0 % Na2S/1.5 % Ca(OH)2Ratio CII: – 3.0 % Na2S/2.0% Ca(OH)2,Ratio CIII:– 3.0 % Na2S/2.5 % CA(OH)2,Ratio CIV:– 3.0 % Na2S/3.0 % Ca(OH)2,CTL: -3.0 % Na2S/3.0 % Ca(OH)2,E – Wastewater from commercial TanneriesFor CI ratio, sample skins weighed 830g, the quantity of sodium sulphide required was 24.9g while hydrated lime is 12.45g. The same procedure was followed for CII, CIII and CIV ratios in the same float of 200% water. After the unhairing-liming process, degree of unhairing, swelling, plumping and change in pH of the wastewater was determined. Samples wastewater from each ratio (CI – CIV) were also collected after unhairing period of 20 hours. The wastewater was again stored until was required for analysis for the same parameters in order to assess the level of pollutants.
3. Results and Discussion
|
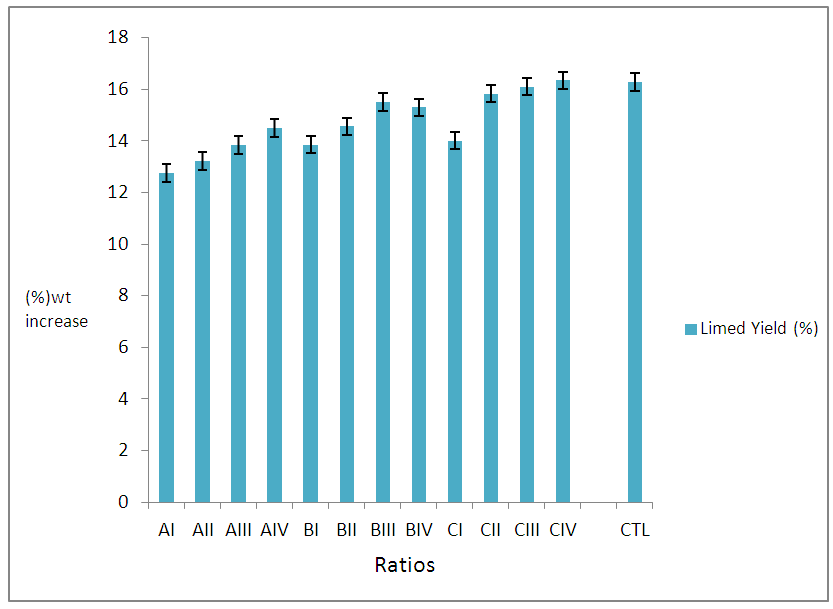 | Figure 1. Effects of Different Ratios of Sulphide/Lime on percentage lime yield |
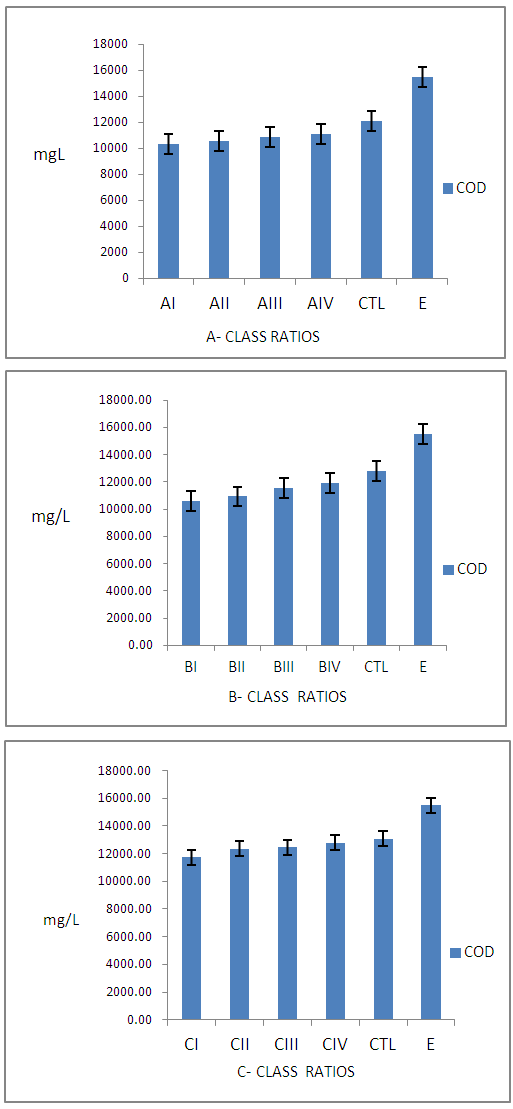 | Figure 2. Concentration of Pollutants Determined by COD from Class A, B, C, CTL Wastewater and Commercial Tanneries samples |
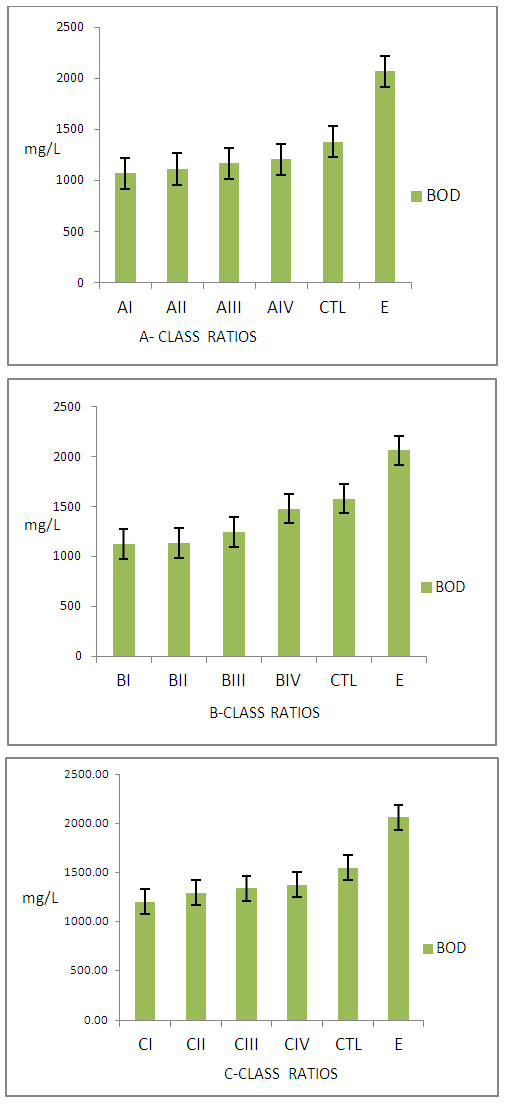 | Figure 3. Concentration of pollutants Determined by BOD from Class A, B, C, CTL Wastewater and Commercial Tanneries samples |
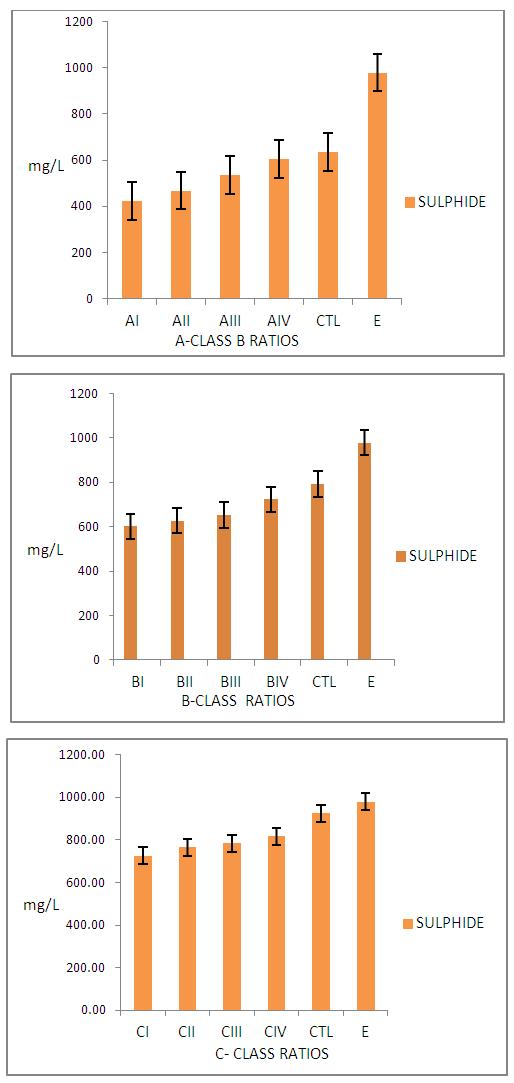 | Figure 4. Sulphide (S2-) Concentration of Wastewater from Class A, B, C, CTL Ratios and Commercial Tanneries samples |
4. Results Description
- Results on degrees of unhairing-liming, swelling and plumping, though by subjective test and percentage weight increase after sulphide-Liming process were observed. The pH of unhairing-liming wastewater indicated, differences depending on the process ratio, (Table 1). It was observed that class ‘A’ ratios did not show effective unhairing and liming, leaving trace of short hairs and other epidermal structures. The class ‘B’ had good unhairing and liming as well as swellings and plumping of the pelts, except for BI. Other ratios in the class indicated very good unhairing and liming results. Finally group ‘C’ ratios gave very good and excellent swellings and plumping on the pelts. Fig. 1 also illustrates lime yield of the various ratios which indicated good results. The wastewater from different groups (A – B) ratios indicates increasing concentration of pollutants with the class ‘A’ ratios having the least and ‘C’ the highest pollution load. All pollutants determined from the wastewater were expressed in mg/L and plotted as shown in Fig.2 - 4 above.
5. Discussions
- Effects of Class ‘A’ Ratios on the WastewaterFig. 2 show the level of pollutants determined by BOD of wastewater from class A - C ratios. It is observed that AI ratio had the least BOD (6068±75.57mg/L), while wastewater from AIII had the highest value (7068±735.17mg/L) in the group. Consequently these values were observed to be below the CTL and commercial tannery sample wastewater with BOD value of 9880.00±406.36mg/L and 11366±792.21mg/L respectively. The COD from commercial tannery wastewater had the highest value (32220±961.12mg/L) while the least was AI (22055±410.36mg/L). It was also observed that COD of wastewater from group A’ study ratios was lower than that of the CTL and the industry as expected. Generally the concentration of pollution load was observed to increase with increase in the percentage of chemicals offered.Sulphide concentration from sample wastewater in class ‘A’ ratios was witnessed to increase from AI - AIV. CTL and sample wastewater from commercial tanneries indicated higher sulphide concentration of 486.33±13.43mg/L and 579±23.87mg/L respectively. This is expected because commercial tanneries are very likely to offer higher quantity of chemicals in order to facilitate chemical reactions to meet the demand of their customers and undermining the effects of these pollutants to the environment.Effects of Class ‘B’ Ratios on the WastewaterFig. 2: shows results of BOD analysis of sample wastewater from class “B”. BI has the least value of 7024±520.88mg/L while BIII having the highest value of 8144±240.67mg/L. Like in group “A,” values of BOD from the commercial tannery and the CTL were higher than the studied ratios except for BIII (8144±240.67mg/L) which was higher than the CTL. This is not expected and might be attributed to the complexity in tannery wastewater [17, 18] or might be due to genetic differences between the raw skins used in this particular experiment [19, 20].For COD, BI had value of 22107.67±1301.24mg/L as the least while ratio BIV had the highest value of 23120.33±949.29mg/L. COD values for CTL sample effluent and that of industry had higher values of 27363.33±988.72mg/L and 32220.00±961.12mg/L respectively indicating higher pollution load. The trend for COD results indicates steady increase (BI - BIV), might be as a result of increase in hydrated lime (1.5 – 3.0%) in the ratios. The use of sodium chloride for preservation of the raw skins and disintegration of sulphur containing amino acids during unhairing may also contribute to high COD, as sulphide requires high quantity of oxygen for its complete oxidation. [21]Sulphide concentration in sample effluent from class ‘B’ ratios show no significant differences in values from BI – BIII, except for BIV as observed in Fig. 2. This is understood, as equal percentages of sodium sulphide (2.8%) was used in this class of ratios. However, the difference observed may be due to complexity of tannery wastewater as reported earlier. The high concentration of sulphide observed in effluent from CTL and that from the industry is indicative of higher offer of the chemicals.Effects of Class ‘C’ Ratios on WastewaterFig. 3 shows concentration of pollutants determined by BOD, COD, Sulphide (S2-,) in wastewater from group ‘C’ ratios. The average BOD value (8464.00mg/L) obtained from the studied ratios is significantly lower than values reported for typical sulphide-unhairing wastewater [14, 22] Sample wastewaters from the commercial tannery had a higher value (11366.00±792.21mg/L) of BOD compared with other class ‘C’. The increase in pollution load of wastewater from this class of procedures is expected because besides increase in percentage of hydrated lime from 1.5 – 3.0% and sodium sulphide from 2.8 – 3.0%, the increased saponification and dissolution of more organic matter from the skins could increase the BOD values.For COD in this group, it is observed that effluent from CI ratios was the least value of 23137.33±852.8mg/L while, CIV 25337.33±762.81.mg/L as the highest. However it can be observed that the wastewater from CIV and CTL has insignificant difference in COD values of 25337.33±252.81mg/L and 25363.33±537.54mg/L respectively. This is expected as the same ratios were used for both CTL and CIV. The COD value from the commercial tannery (32220.00±961.12mg/L) wastewater has consistently observed to be higher than other classes even the ‘C’ class studied ratios.From Fig. 3 the concentration of sulphide in wastewater shows little differences between the class ‘C’ ratios. This suggests that only optimal percentage of chemicals required for the process of unhairing-liming was actually used, the excess goes into the wastewater. Meanwhile, wastewater from CIV method remains higher in sulphide concentration (487.67±26.84mg/L) than those from the same group. But the average sample effluents from commercial tannery had 579±46.54mg/L higher than those in the entire class. The fluctuations noted of this pollutant in the class ‘C’ ratios may be attributed to genetic differences from the skins as earlier observed, the sample skins could be more hairy.Preparation of unhairing-liming ratios has shown good prospects for the reduction of pollution load in the unhairing-liming process in the manufacture of upper leathers. Although the quality of the final leathers are yet to be determine, it is not expected to be lower than the standard specifications. This approach has reduced pollutants in the tannery wastewater and is likely to lessen the cost of treatment chemicals. The difficulty often experience in the handling of large volumes of complex liquid waste will also be eased, particularly from the unhairing-lime process that generates most noxious waste in any given tannery [23-25].
6. Conclusions
- Results of pollutants determined from wastewater generated from the individual studied ratios indicated that BII and BIII actually had lower pollutants compared to the CTL and commercial industry. It was observed that the prepared ratios led to percentage reduction of pollutant determined by COD to 19.16% for BII while ratio BIII indicated 22%. For BOD reduction, it was found to be 24% and 17.6% for BII and BIII respectively. It is imperative to recommends the said ratios for adoption as optimal for unhairing and liming of goat skins for the production of upper leathers. As the wastewater produced in the use of these ratios was observed to constitute fewer pollutants.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML