-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2018; 8(3): 57-64
doi:10.5923/j.chemistry.20180803.01

Lactic Acid Production from Sisal Boles Juice by Lactobacillus Delbrueckii Sp. Delbrueckii
N. Msuya, J. H. Y. Katima, E. Masanja, R. J. A. Minja, A. K. Temu
Department of Chemical and Mining Engineering, University of Dar es Salaam, Dar es Salaam, Tanzania
Correspondence to: N. Msuya, Department of Chemical and Mining Engineering, University of Dar es Salaam, Dar es Salaam, Tanzania.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

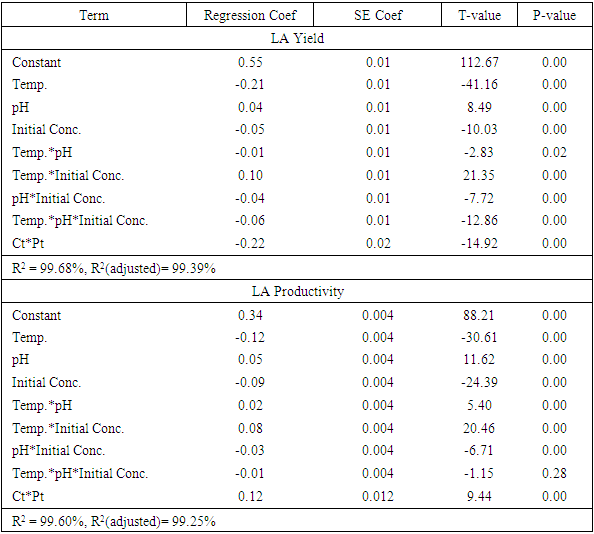
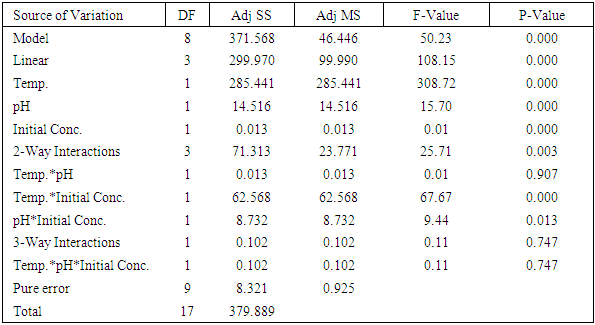
The use of different concentrations of sugar from sisal boles juice for production of Lactic acid (LA) using Lactic Acid Bacteria (LAB) has been studied with the intention of analysing the effect of initial sugar concentration, process temperature and initial medium pH on the produced lactic acid concentration, yield and productivity. All the linear variables (initial sugar conc., pH and temperature), two way and three way interactions were statistically significant for LA yield at p-values of less than 0.05 with correlation coefficient of 0.997. There was no significant effect on LA productivity of three way interactions (Temp.*pH*Initial Conc.). Maximum condition for production of LA occurred at a temperature of 37°C, initial pH of 6 and initial sugar concentration of 120 g/L which corresponded with the highest LA concentration of more than 24 g/Land a yield of 93%. This study shows that sisal boles juice has potential to produce LA.
Keywords: Sisal boles, Juice extraction, Lactic acid production, Delbrueckii sp. Delbrueckii, PLA
Cite this paper: N. Msuya, J. H. Y. Katima, E. Masanja, R. J. A. Minja, A. K. Temu, Lactic Acid Production from Sisal Boles Juice by Lactobacillus Delbrueckii Sp. Delbrueckii, American Journal of Chemistry, Vol. 8 No. 3, 2018, pp. 57-64. doi: 10.5923/j.chemistry.20180803.01.
Article Outline
1. Introduction
- Lactic acid (2-hydroxy propanoic acid) is the simplest hydroxyl acid with an asymmetric carbon atom and exists in two optically active configurations, levorotatory (L) and dextrorotatory (D) [1-4]. Traditionally it was only used in food as preservative, pharmaceutical and leather tanning industries but recently new applications as a building block for renewable and biodegradable plastics, poly (lactic acid) (PLA) polymers has been found [5]. The worldwide demand for LA is over 150,000 tonnes per year [6] and is expected to increase rapidly as industrial feedstock for PLA production [7, 8, 9]. Komesu et al. (2016a) forecasted the annual demand to be 400,000 tonnes by the year 2017. With the increase of 5-8% per annum as projected by Yadav et al. (2011) the annual demand will be more than 1.0 million tonnes before 2025. The global annual production for LA was estimated to be 130,000–150,000 tonnes [10]. LA can be synthesized industrially by either chemical or biological routes. Chemical synthesis is mainly based on the hydrolysis of lactronitrile by strong acids like concentrated sulphuric acid or hydrochloric acid [11]. This route yields only dextrorotatory-levorotatory lactic acid (DL-LA), the mixture of L(+) and D(-) lactic acid (racemic mixture of the two isomers) [6, 12-14]. The biological route is through fermentation of starch and other polysaccharides [3, 15-18]. Biological route has the advantage of producing optically pure L(+) or D(-) lactic acids because specific microorganisms, substrates and conditions can be selected [6, 11, 19]. LA with high optical purity is required for the production of PLA [17, 18].The most serious obstacle for LA production commercially is the availability and cost of feedstock for fermentation [20]. Different materials have been used for production of LA like pure sugars and food crop sources like potatoes, cassava, corn, wheat, rice, sugar beet and sugar cane [11, 13, 17, 21-26]. Utilization of pure sugars and food crops as carbon source in lactic acid production is economically unfavourable, because of competition with existing uses [27, 28]. The utilization of non-food sources and cellulosic materials is a promising approach since they are abundant, renewable, relatively cheap and do not compete with food [12, 29, 30]. Industrial wastes, agricultural waste and forestry waste have been recommended as cost effective feedstocks for large scale fermentation [6, 21, 28, 29, 31]. However, cheap, non-food, and renewable raw materials still need to be identified to reduce the production cost of lactic acid and hence PLA [29, 32]. Material like sisal boles with total sugars of 26-30% can be used to extract juice that can be fermented to produce LA [33]. Little or no work has been done to produce LA from this huge resource. This work analyses the effects of initial sugar concentration, process temperature and initial medium pH on the production of lactic acid from sisal boles juice.
2. Material and Methods
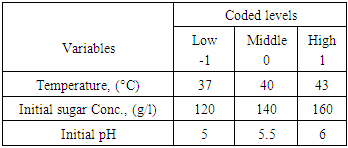
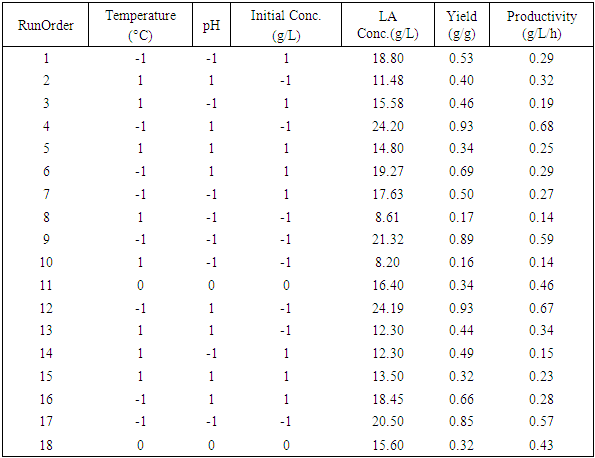
- The raw material used was juice from sisal boles (Agave - Hybrid 11648). The juice extraction process involved chopping of sisal boles into small pieces (about 5-15 cubic millimetre) to increase surface area during pressing. Chopped boles were pressed using a manual pressing machine with hydraulic pressure which was combined with filtration using filter cloths. The juice was then hydrolysed to break the sugars into fermentable sugars. The hydrolysis method by Masalla (2010) was adapted with slight modification. The pH of the juice was reduced to 1.0 using concentrated hydrochloric acid (36%) and then heated in oil bath at a temperature range of 60-100°C for 30-60 minutes to complete the thermo-acid hydrolysis. The hydrolysates were then left to cool to room temperature (30°C±2). The procedure for preparation of stature culture by Panesar (2010) was adapted with slight modification. The microorganism strain utilised was Lactobacillus delbrueckii WLP677 from the White Labs Inc. (CA 92126 USA). The bacterial culture was grown in 50ml of De Man, Rogosa and Sharpe agar (MRS) medium in 250 ml Erlenmeyer flask [16]. The prepared MRS medium contained the following (g/l): peptone, 10; meat extract, 5.0; yeast extract, 5.0; dextrose, 20; potassium phosphate, 2.0; tween 80, 1.0; tri-ammonium citrate, 2.0; magnesium sulphate, 0.05; sodium acetate, 5.0 and agar, 12. After sterilization at 121°C for 15minutes using portable steam Autoclave (Heuer, 220V and 50Hz) and cooling to room temperature (30°C±2), the medium was inoculated with about 10% of cells from agar stab. The medium was then incubated at 37°C for 24 hrs under stationary conditions for microbial growth before fermentation. The pH of the fermentation medium was regulated before fermentation to 5 or 6, using sodium hydroxide (12.5M = 50%). A 23 full factorial design was used to study the influence of three parameters: pH, initial sugar concentration and medium fermentation temperature on the responses (LA concentration, yield and productivity). The real and coded variables are presented in Table 1.
|
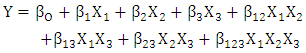 | (1) |
 | Figure 1. Calibration Curve for LA Concentration determination |
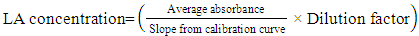 | (2) |
3. Results and Discussion
- The calibration curve gave a linear relationship with R2 of 0.999 and the fermentation results are presented in Table 2.
|
|
 | (3) |
|
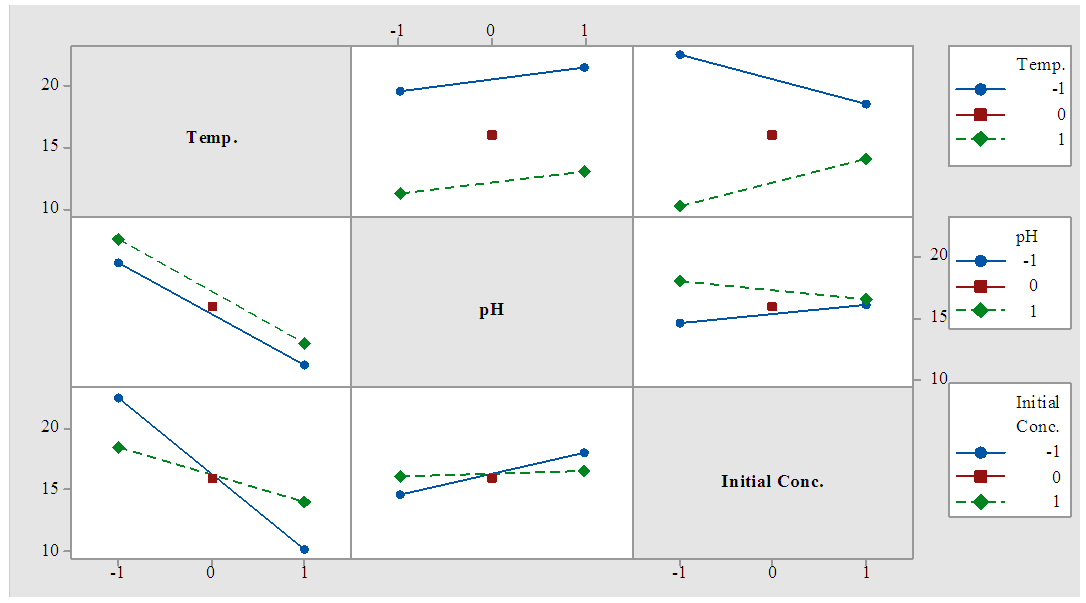 | Figure 2. Interaction plot for LA concentration (data means) |
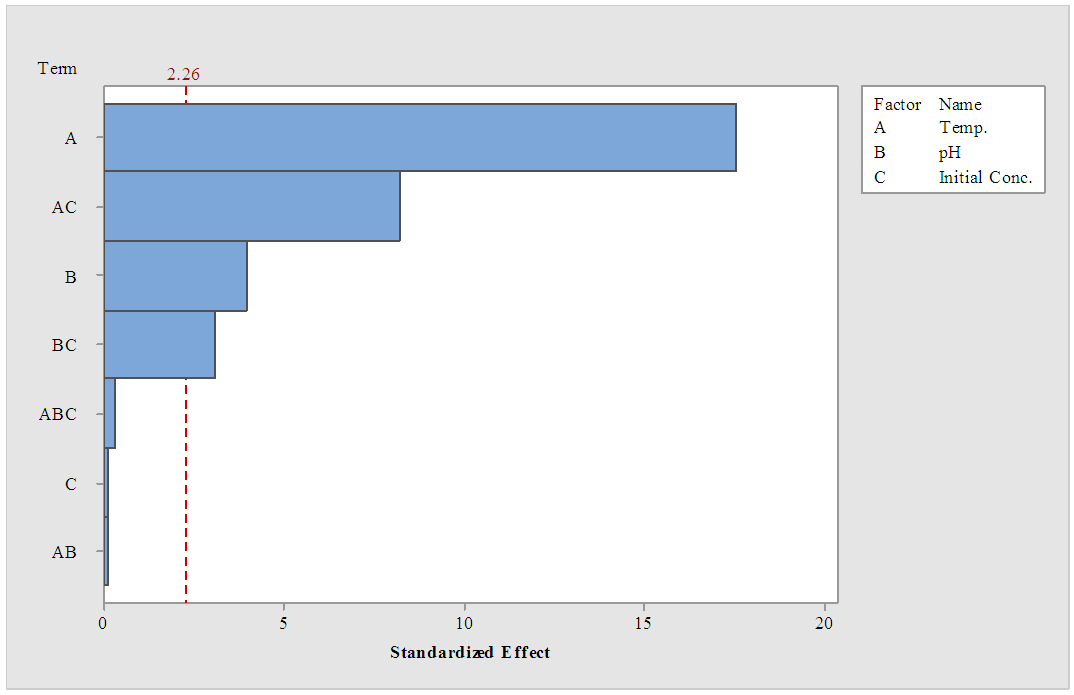 | Figure 3. Pareto chart for LA concentration at α =0.05 |
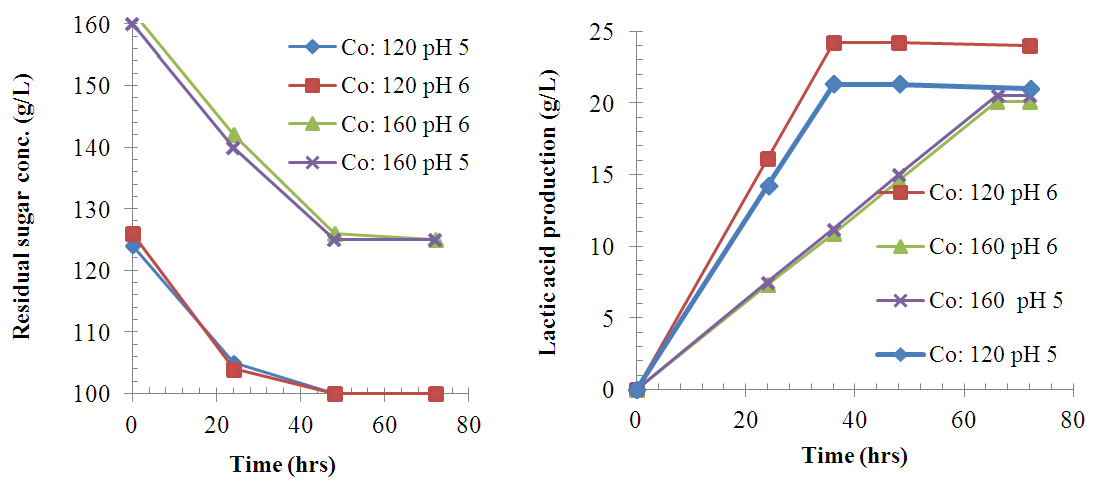 | Figure 4. Variation of sugar concentration (Co) and LA production with time during fermentation at 37°C |
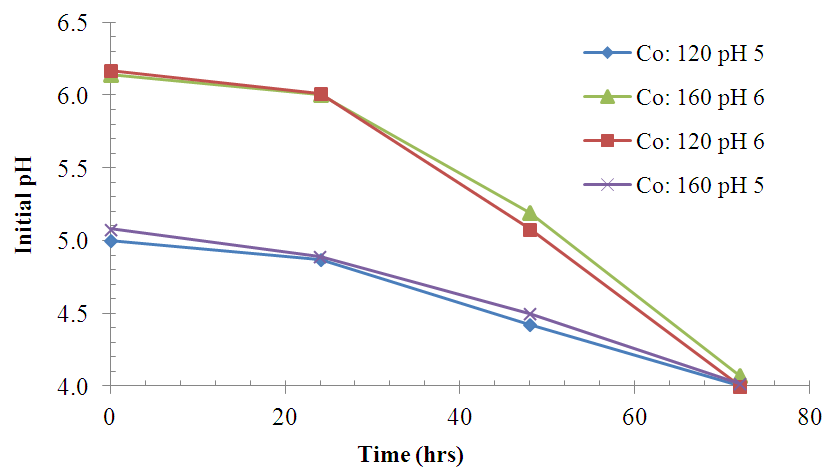 | Figure 5. Variation of pH with time during fermentation at 37°C |
4. Conclusions
- 93% of the sugar available in sisal bole juice could be converted to LA through fermentation using LAB. All the linear variables (initial sugar conc., pH and temperature), two-way and three-way interactions were statistically significant variables for LA yield at p-values of less than 0.05 with correlation coefficient of 0.997. There was no significant effect on LA productivity of three-way interactions (Temp.*pH*Initial Conc.). Using interaction plots on LA concentration at α = 0.05, the great departure of lines from parallel occurred when combining temperature with initial sugar concentration, and pH with initial concentration. This indicates that LA concentration at temperature or pH level depends upon the initial sugar concentration levels. There was no effect on LA concentration when combined pH and Temperature. In addition, the largest standardized effect on LA concentration was temperature because it extended the Pareto chart reference line the farthest. Maximum production of LA (24g/L) from sisal juice using Lactobacillus delbrueckii WLP677 bacteria occurred at temperature of 37°C, pH of 6 and initial sugar concentration of 120 g/L. Higher temperature of 43°C with similar sugar concentration and pH produced about 50% less LA. This study confirms the fact that sisal boles juice can be used to produce LA through microbial route. Further studies on the optimization of LA production (concentration and yield) from sisal boles juice are recommended.
ACKNOWLEDGEMENTS
- We are gratefully for the financial support by Tanzania Commission for Science and Technology (COSTECH) under Bioplastics Project and DAAD - in Country scholarship through University of Dar es Salaam (UDSM) -Tanzania. Special thanks to Mr. Abdi A. for his support during data collections.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML