-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2018; 8(2): 51-56
doi:10.5923/j.chemistry.20180802.05

Cloud Point Extraction Using BBTSC Reagent for Pre-concentration and Determination of Zinc in Some Environmental Water Samples by Flame Atomic Absorption Spectrometry
Rehab E. Babiker, Mohamed A-Z Eltayeb
Department of Chemistry, Faculty of Science and Technology, Al- Neelain University, Khartoum, Sudan
Correspondence to: Rehab E. Babiker, Department of Chemistry, Faculty of Science and Technology, Al- Neelain University, Khartoum, Sudan.
| Email: |  |
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

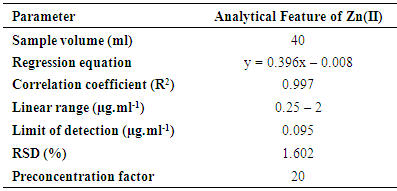
A simple and convenient method based on cloud point extraction was proposed for the determination of zinc. The extraction was carried out in the presence of benzilbis (thiosemicarbazone) (BBTSC) as complexing agents and octylphenoxypolyethoxyethanol (TritonX-114) as the non – ionic surfactant. The factors affecting the cloud point extraction were optimized. Under the optimum conditions, the calibration graph was linear in the range of (0.25 – 2.00 μg ml-1) with a detection limit of 0.095µg.ml-1. The relative standard deviation for 7 replicates of 0.5 µg ml-1 Zn(II) was 1.602%. The stoichiometry was 1:1, Zn (II): BBTSC. The method was applied to the determination of Zn(II) in various water samples with satisfactory results. The preconcentration factor for Zn(II) calculated by dividing the aqueous phase volume by the final volume of diluted surfactant rich phase was 20.
Keywords: Zinc(II), Cloud point extraction, Benzilbis (thiosemicarbazone), Water samples, Triton X – 114
Cite this paper: Rehab E. Babiker, Mohamed A-Z Eltayeb, Cloud Point Extraction Using BBTSC Reagent for Pre-concentration and Determination of Zinc in Some Environmental Water Samples by Flame Atomic Absorption Spectrometry, American Journal of Chemistry, Vol. 8 No. 2, 2018, pp. 51-56. doi: 10.5923/j.chemistry.20180802.05.
Article Outline
1. Introduction
- Zinc is essential element for all animals as well as human beings. It has a crucial physiological role in human blood which distributed by 75-85% in erythrocytes 12-22% in plasma and 3% in leukocytes [1-3]. As Zn(II) is both toxic and essential element, so it is necessary to monitor and control the trace balanced level of it. Thus highly and selective methods are still required for trace determination Zn(II) in different biological and pharma samples.Cloud point extraction has been used for the extraction of metal ions after the formation of sparingly water – soluble complexes [4-7]. Zn(II) was determined by flame atomic absorption spectrometry after cloud point extraction using complexing agents [8].CPE methodology is chosen as rapid sensitive method for extraction of many of element cations from aqueous solution after formation complexes with selective organic reagents coupled with spectrophotometric method for determination metals in vital and environmental samples [9]. Cloud point extraction CPE is depending on phase behavior of surfactants in aqueous solution and exhibit phase separation at specific temperature. Separation and pre-concentration according to (CPE) are becoming an increasing important in analytical chemistry [10, 11]. In this paper a CPE method for pre-concentration of Zn(II) was proposed.
2. Materials and Methods
2.1. Preparation of Benzilbis (Thiosemicarbazone) [12]
- The reagent (BBTSC) was prepared by the Sulekh Chandra et al procedure.(10.5 g. 1 mmol) of benzil was dissolved in 50 ml of ethanol (99.9%), and (9.1 g. 2 mmol) of Thiosemicarbazide was dissolved in 50 ml of ethanol (99.9%). The reaction mixture was heated under reflux for 3 hours, then the solvent evaporated about a half and a yellow crystalline solid was formed, which was filtered, recrystallized with ethanol, and dried at room temperature (m. p. 165°C).
2.2. Preparation of Reagents
- All chemical reagents used were of analytical reagent grade, and deionized water was used throughout the experiments. Stock Zn(II) solution was prepared by dissolving appropriate amounts of Zn(NO3)2.6H2O (Merck) in deionized water, working standard solution was obtained daily by stepwise dilution of the standard stock solution. Triton X–114 stock solution (5%, V/V) was prepared by dissolving 5ml of concentrated solution (Merck 100%) in deionized water. Benzilbis (Thiosemicarbazone) (BBTSC) solution in Dimethyl formamide (LOBA Chemie 99.8%) was prepared. A stock buffer solution was prepared by dissolving appropriate amounts of citric acid monohydrate 0.1M (Scharlau Chemie S. A) and disodium hydrogen orthophosphate dihydrate 0.2M (s. d fine – CHEM LTD) in deionized water. Ethanol Absolute (Scharlau 99.9%).
2.3. Instrumentation
- A flame atomic absorption spectrometer, (GBC SavantAA Version 3.02, Australia) equipped with deuterium background correction, and air – acetylene flame was used for zinc measurements. Zinc hollow cathode lamp was used as the radiation source. HH-S THERMOSTATIC WATER BATH was used to maintain the samples at the desired temperature. eppendorf centrifuge 5804R, Germany was used to accelerate and separate phases. A digital pH meter (Metrohm, model 691) with a combined glass electrode was applied to measure pH values.
2.4. Procedure
- An aliquot of 40ml of a solution containing 0.5 µg/ml zinc nitrate and 0.8ml of 0.25% of (BBTSC ) was adjusted to pH 8 with 8ml of buffer solution (citric acid and disodium hydrogen orthophosphate), 0.8 ml of 5% (V/V) Triton (X–114) was added, the mixture was shaken for 1min and left to stand in a thermostatedbath at 50°C for 15min. Separation of the phases was achieved by centrifugation at 3000rpm for 10min. The whole system was cooled in an ice bath for 30 min. so that, the surfactant rich phase would regain its viscosity. In this way, the bulk aqueous phase was easily decanted. Theremaining micellar phase was dissolved in 5ml of 1M HNO3 in ethanol and then the Zn(II) ions content was readily evaluated by FAAS.
2.5. Stoichiometry of Zn(II) Complex
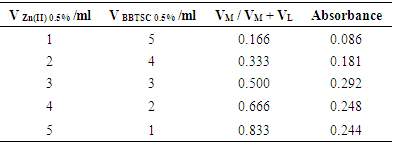
- A series of solutions were prepared by mixing the indicated amount of both 0.01M solutions of Zn(II) and BBTSC. Volumes of 1, 2, 3, 4 and 5 ml of 0.01M of Zn(II) solutions were placed into five 50ml measuring cylinders, followed by addition of 6ml of buffer solution pH 8.5, and 5, 4, 3, 2 and 1 ml of 0.01M BBTSC solutions, respectively and diluted to 40ml by de ionized water. the solutions were shaking for 1min. and the general procedure of section 2.4.4.1 was used with the optimum conditions. The results were shown in and Figure (3.6).
3. Results and Discussion
- The yield of CPE is dependent on various factors including, concentration of surfactant, concentration of reagent, electrolyte concentration, temperature and pH. So as to achieve maximum efficiency, absorbance and sensitivity, the aforementioned factors was investigated and optimum conditions were obtained.
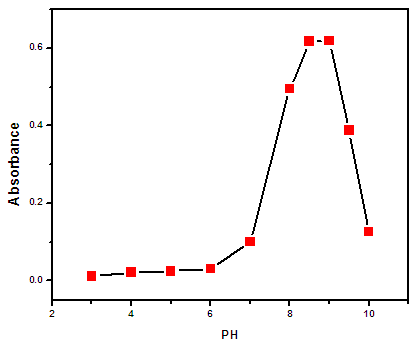
3.1. Effect of pH
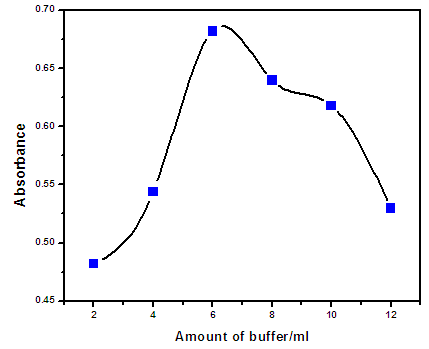
- The extraction of metal ions by the CPE method involves the formation of a metal-chelate with sufficient hydrophobicity to be extracted into the small volume of the surfactant-rich phase [13]. The pH plays a unique role in metal-chelate formation and in subsequent cloud point extraction yield [14]. Therefore, pH was the first parameter evaluated for its effect on the determination of zinc ions. Figure (3.1) shows the effect of pH in the range of 3 - 10 on the extraction of the Zn(II) complex. Quantitative extraction efficiency was achieved at pH 8.5. For this reason, pH 8.5 was used for the subsequent work.Also the effect of buffer solution volume at the fixed pH value was studied in the range of 2 - 12 ml in order to improve analytical sensitivity. The maximum absorbance was obtained for a buffer volume of 6ml as shown in Fig (3.2).
3.2. Effect of Benzilbis (Thiosemicarbazone) Concentration
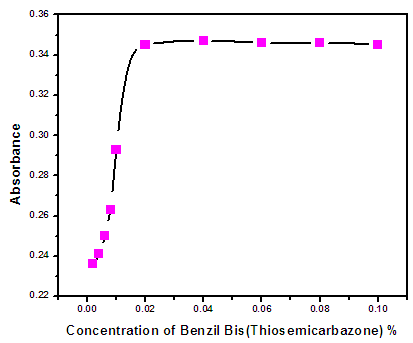
- Forty milliliters of a solution containing 0.5 μg of Zn(II), 0.4% Triton X-114 at pH 8.5 containing various amounts of BBTSC in the range of 0.002 – 0.1% were subjected to the cloud point pre concentration process.. Fig (3.3) Shown that the absorbance increased from (0.002 – 0.02)% and remained almost constant with further increase in BBTSC concentration up to 0.02%. Therefore, BBTSC concentration of 0.02% was employed for further experiments.
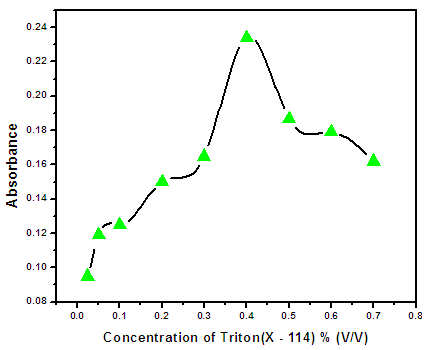
3.3. Effect of Triton X-114 Concentration
- Triton X-114 is one of the non-ionic surfactant extensively used in CPE. This is due to its advantages such as commercial availability with high purity, relatively low cloud point temperature, low toxicity and cost and high density of the surfactant-rich phase which facilitates phase separation by centrifugation. Fig (3.4) Shows the effect of non-ionic surfactant concentration within the concentration range of 0.025–0.7% (v/v), on the CPE efficiency of Zn(II) ions. The absorbance of the complex was increased by increasing the Triton X-114 concentration up to 0.4% (v/v). A considerable decrease in the absorbance is observed with increasing surfactant amounts higher than 0.4% (v/v). This can be attributed to an increase in volume and viscosity of the micellar phase. At concentrations below this value, the extraction efficiency of complexes was low because there are few molecules of the surfactant to entrap the BBTSC complexes quantitatively. Thus, Triton X-114 concentration of 0.4% (v/v) was selected for subsequent experiments.
3.4. Effect of Equilibration Temperature and Incubation Time
- The CPE technique is based on the property of most non-ionic surfactants in aqueous solutions to form micelles and become turbid when heated to cloud point temperature. The effect of the equilibration temperature above the cloud point temperature was investigated within a range of 40 to 70°C. It was found that an equilibration temperature of 60°C is adequate to obtain quantitative extraction as shown in Fig (3.5). The incubation time was a parameter also considered. At 60°C, it was observed that an equilibration time of 20 min is sufficient to obtain a good extraction.
3.5. Effect of Centrifuge Time and Rate
- The influence of centrifuge time within range of (5 -35 min) at various rates (2000-5000 rpm) on phase separation procedure and method sensitivity was examined. It was found that 15 min centrifuge time at 4000 rpm is sufficient for complete quantitative phase separation.
3.6. Effect of Ionic Strength
- It is known that ionic strength of the solution is one of the effective factors in cloud point extraction. In this work, the effect of NaCl salt as an electrolyte, on the process was investigated. It is observed that the increasing concentration of salt up to 0.01% has no effect on the process, but more concentration caused decreasing in absorbance signal of extracted surfactant-rich phase.
3.7. Analytical Figures of Merit
- Under the optimal conditions, the analytical performance of the proposed method was studied. The calibration graph was linear from 0.25 to 2 µg·ml-1 with a correlation coefficient of 0.997. Table (3.1) summarizes the analytical characteristics of the optimized method, including regression equation, linear range, and limit of detection, reproducibility, and preconcentration factor. The limit of detection, defined as CL=3SB/m (where CL, SB, and m are limit of detection, standard deviation of the blank, and slope of the calibration equation, respectively) was 0.276 µg.ml-1. The preconcentration factor calculated as the ratio of the concentration of the analyte after preconcentration to that before preconcentration. The relative standard deviation (R.S.D%) for seven replicate measurements of 0.5µg.ml-1 zinc was 1.602%.
|
3.8. Effect of Foreign Ions
- In order to evaluate the selectivity of the method, the effects of different ions on the solid phase extraction and determination of zinc were investigated. A constant concentration of zinc(II) (0.5 µg ml-1) was taken with different concentrations of ions and the general procedure was followed. Results indicate that the proposed method is selective for the determination of zinc(II) when used in connection with AAS but there will be interference when used with UV-Vis spectrometry.
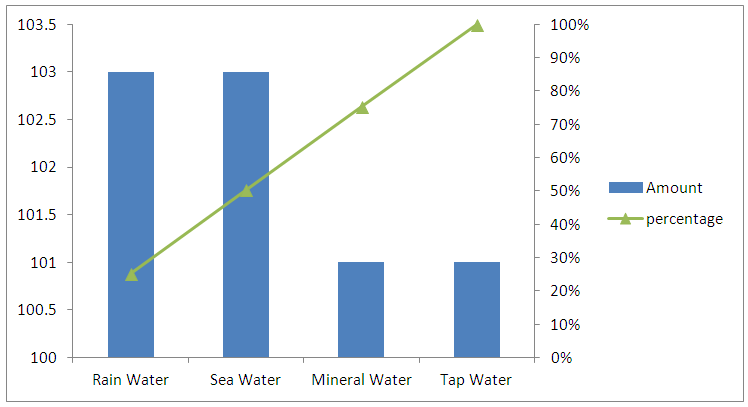
3.9. The Application of the Developed Method
- In order to evaluate the analytical applicability of the proposed method, it was applied to the determination of zinc in several water samples spiked with standard Zn(II). Water samples were filtered using Whatman filter paper to remove suspended particulate matter and stored in refrigerater in the dark. Three replicate determinations were carried out and the obtained results (Table 3.2) were satisfactory. Additionally, Table 3.2 shows high percentage recovery of the method. Recovery was calculated as follows:R(%) = [ ( Cm – C0 )/m ] * 100Where Cm (µg ml-1), C0 ( µg ml-1 ) and m are values of zinc measured in spiked sample, in unspiked sample, and amount of zinc added (µg ml-1), respectively.
|
|
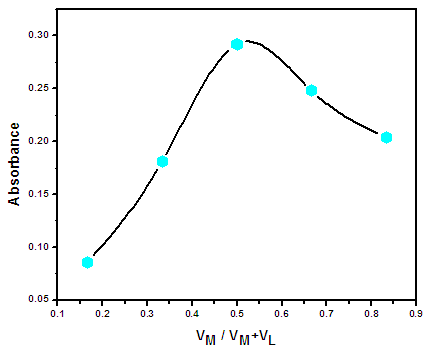
3.10. Stoichiometry of Zn(II) Complex
- The stoichiometry of Zn(II) complex was carried out by Job’s continuous variation method. The result shown in Table (3.3) and Figure (3.6) indicated that the ratio were found to be (1: 1) (metal: ligand) for Zn(II) complex.
4. Conclusions
- The purposed cloud point extraction method using BBTSC ligand as a stable and fairly selective complexing agent offers a simple, rapid, inexpensive and environmentally benign methodology for preconcentration and separation of Zn(II) in aqueous solutions. This method gives very low LOD, good RSD and it was applied to determine trace amounts of Zn(II) ions in various water samples. In a full comparison of presented results in this paper with those previously reported, it is found that this method is superior in terms of linear range, detection limits and selectivity.
 | Figure Pareto chart…. |
 | Figure 3.1. Effect of pH on the absorbance of Zn(II). Conditions: 0.5µg /ml Zn(II), 0.005% BBTSC, 0.1% (v/v) Triton (X – 114), 15 min heating at 50°C, 10 min centrifuge in 3000 rpm in various pH |
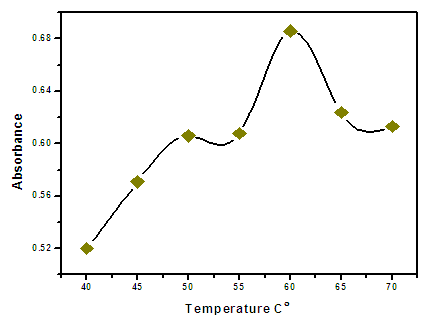 | Figure 3.5. Effect of equilibration temperature on the analytical signal of Zn(II). Conditions: 0.5 µg/ml Zn(II), 6 ml of buffer solution pH 8.5 |
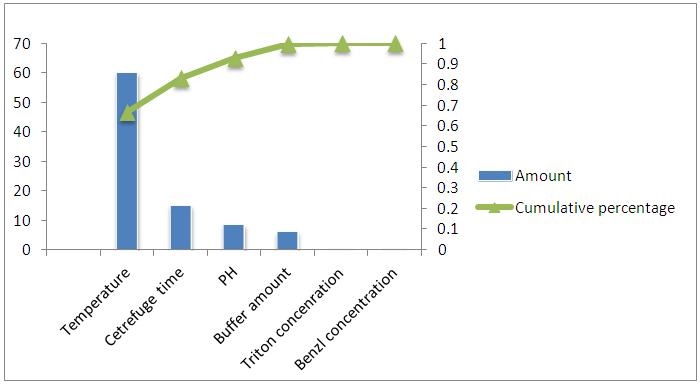 | Figure Pareto chart…. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML