-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2018; 8(2): 45-50
doi:10.5923/j.chemistry.20180802.04

Thermal-Acid Hydrolysis of Sisal Boles Juice for Lactic Acid Production
N. Msuya, J. H. Y. Katima, R. J. A. Minja, E. Masanja, A. K. Temu
Department of Chemical and Mining Engineering, University of Dar es Salaam, Dar es Salaam, Tanzania
Correspondence to: N. Msuya, Department of Chemical and Mining Engineering, University of Dar es Salaam, Dar es Salaam, Tanzania.
| Email: |  |
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The effect of reaction temperature, time and pH on the maximum total sugar concentration during thermal-acid hydrolysis of sisal boles juice was investigated. The Minitab software (V. 17) was used to design a 2n full factorial design for sisal juice hydrolysis and analyse the effects of the variables on the response. Thermal-acid hydrolysis can be carried out at a moderate temperature of 60°C with a concentrated hydrochloric acid at pH of one within a period of 30 minutes to achieve a sugar yield of 39%. The breakdown of complex sugars to simple sugars of sisal bole juice is basically a function of acid concentration in sugar solution. Lowering pH of the juice contributed to increase in hydrolysis rate. The use of ANOVA analyses gave evidence that linear, 2- way interactions and 3-way interactions of all the variables: temperature (60-100°C), time (30-60 min) and pH (1-5) significantly affected the sugar yield. This amount of sugar yield from sisal boles juice is higher than the yield of 26% which has been reported. The hydrolysed juice was also subjected into fermentation for lactic acid and results showed favourable LA yield was obtained with the recommended thermal-acid hydrolysis in this study.
Keywords: Thermal-acid hydrolysis, Sisal bole juice, Complex sugars breakdown, Lactic acid production
Cite this paper: N. Msuya, J. H. Y. Katima, R. J. A. Minja, E. Masanja, A. K. Temu, Thermal-Acid Hydrolysis of Sisal Boles Juice for Lactic Acid Production, American Journal of Chemistry, Vol. 8 No. 2, 2018, pp. 45-50. doi: 10.5923/j.chemistry.20180802.04.
Article Outline
1. Introduction
- Sisal is a semiarid and marginal land crop of the tropics whose leaves are used for extraction of fibres. In a typical sisal production process, a very small portion, about 2% of sisal plant (sisal fibres) is used to produce twines, packaging’s, carpets, marts, threads, fine yarns, ropes and roofing tiles. The remaining bulk (98%) which include leaves decortications residue and sisal postharvest remaining (sisal poles, sisal boles with leave stubs) is discarded as waste [1-3]. Sisal fibres have also been used in the automotive sector and for specialist paper manufacturing [4]. Generally, production of one tonne of fibre generate waste amounting to 24 tonnes of leaf residues, 100 m3 of wastewater and 4.7 tonnes of sisal boles [4]. On average a sisal bole weighs 40 kg and contains juice which consist of hydrolysable sugars (mostly fructose) that can be fermented to produce different bio-products such as citric acid, lactic acid and ethanol [1, 2, 5].Extraction of juice from sisal boles is discussed in earlier study by Msuya et al, [5]. Before fermentation to other bio-products the extracted sisal bole juice need to be hydrolysed to break down the total sugars into simple monomer sugars that can be assimilated into microbial cells. Various researchers have worked on different methods for breaking down the complex sugars in sisal boles into simple sugars [2, 6, 7]. Conversion process of polysaccharides (mainly fructans) to fermentable sugars from sisal juice can be done either by thermal, enzymatic or chemical hydrolysis. Enzymatic hydrolysis leads to high purity fructose yield but requires commercial hydrolytic enzymes such as fructanase which are expensive considering high volume production [2]. The enzymatic hydrolysis is also very slow [8]. In addition, some hydrolysis products such as glucose tend to inhibit the process and thus need to be removed, this increases complexity of the process [2].Thermal hydrolysis involves heating the chopped boles to temperatures above 100°C by autoclaving or oven cooking. The boles are cooked or autoclaved to hydrolyze the fructans and release fermentable sugars, principally fructose [2, 9]. Here the hydrolysis is done before extraction of juice. Thermal hydrolysis has the advantage of ease of controlling, acceptable processing time and relatively low capital investments costs [2, 10]. The resulting fructose syrup has a relatively low purity and can form Maillard reactions due to high temperatures (above 100°C) used [1, 2]. A Maillard reaction is a non-enzymatic reaction between sugars and proteins that occurs upon heating and that produces browning colour into the product. These are fermentation inhibitors which reduces the microorganism activities hence low product yield [10-12].Acid hydrolysis (dilute or concentrated) involves use of large quantities of mineral acids, which has to be recovered, resulting in high cost of neutralization and gypsum disposal problem [13] if sulphuric acid is used. Unlike dilute acid hydrolysis, concentrated acids gives higher sugar yields with fewer degradation products which can favours microbial growth hence minimize inhibition during fermentation [11, 14, 15]. Combined acid and medium temperature hydrolysis (thermal-acid hydrolysis) can compromise use of larger quantities of mineral acid and the use of excessive heat applications [2, 16, 17] that can lead into production of fermentation inhibitors such as hydromethylfulfaral. This work investigated the effect of reaction temperature, time and acid concentrations on the sugar yield during thermal-acid hydrolysis of sisal boles juice.
2. Material and Methods
2.1. Extraction of Juices from Sisal Boles
- Samples of Agave H11648 boles 10-12 years old were randomly collected from one hectare of Katani Ltd sisal estate, Tanga, Tanzania. The boles were weighed before and after removing the leaf stubs. They were then cleaned using tap water. Juice extraction process involved chopping of sisal bole into small pieces (about 5-15 mm3) to increase the surface area during pressing. The juice was extracted from 1 kg sample of chopped boles using hydraulic pressing machine with a capacity of 16 tonnes. The extraction machine is made of stainless steel housing. Three alternatives were tried to maximize juice extraction. The first option involved direct pressing of chopped sisal boles without heating, the second involved heating the boles by autoclaving at 121°C for 5 minutes. The autoclaved chopped bole parts were then cooled to room temperature (30°C ± 3) before pressing. The third option involved mixing the boles with 1L of boiled water (100°C) before pressing to increase solubility of soluble components in bole mass to bring about the easiness during extraction process. The biomass residue after the first juice collection in either option was tested for residual sugar concentrations. This was done by adding half a litre of hot water to enhance wet blending of the biomass residue and pressed to extract the juice to be tested. The process was repeated to ensure minimal sugar residual in the pulp by measuring sugar content of the wash liquor extract.
2.2. Hydrolysis of Sisal Boles Juice
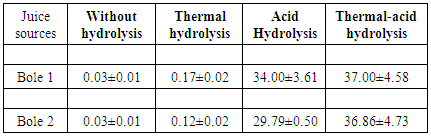
- Three options were tested for the suitable hydrolysis method on the sugar yield as per literature information. The first option involved autoclaving the juice at 121°C (thermal hydrolysis) for 15minutes without acid. The second option involved acid hydrolysis (pH 1-2) without heating and the third option combined both acid and temperatures (thermal-acid hydrolysis) for 15 minutes at 121°C. Total sugars concentrations were determined using UV Light Spectrophotometer at 540nm (SPECTRONIC 21D, MILTON ROY) by DNS method [18]. The calibration standard curves were drawn using standard sugars from Sigma Aldrich. The sugar concentrations were calculated using equation (i).
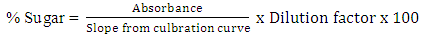 | (1) |
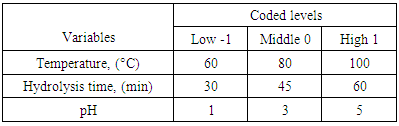
2.3. Design of Experiment for Thermal-Acid Hydrolysis
- To maximize sugar extraction from the extracted juice through hydrolysis, the design of experiment was done on thermal-acid hydrolysis to identify the possible combination that could favour high sugar yields fermentable to lactic acid. The thermal-acid hydrolysis process involved transferring of about 50 ml of juice into 100 ml flat bottomed conical flasks followed by adjustment of the solution pH between the ranges of 1-5 using concentrated hydrochloric acid (36%). The low and high levels were adapted from reported information from literature [2, 17, 20] and are given in Table 1. The samples were placed in an oil bath at temperature range of 60 to 100°C and time range of 30 to 60 minutes.
|
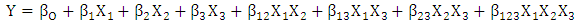 | (2) |
3. Results and Discussion
3.1. Results of Juice Extraction and Sugar Yield on the Tested Hydrolysis
- The calibration curve for the standard sugars gave a linear relationship with R2 of 0.9971. Juice extraction results were 567±2.9, 791±0.6 and 653±2.9 volumes (ml) of juice per kg of sisal bole for chopped and pressed, autoclaved, and mixed with boiling water, respectively. Thus, the autoclaved sisal boles gave the highest volume of juice compared to the other methods. This can be attributed to higher temperature (121°C) used under autoclaving at pressure of 1.5 bar, which led to rapture of sisal boles microfibril structure providing more surface area for juice extraction. The sugar yield results from the tested hydrolysis options are presented in Table 2.
|
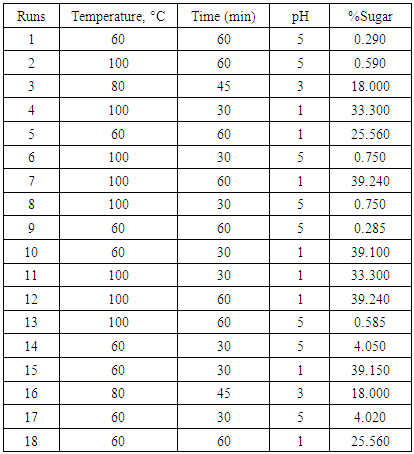
3.2. Effect of Temperature, Time and pH on Sugar Concentration for the Designed Hydrolysis
- The effect of temperature, time and pH on acid hydrolysis of sisal bole juice was investigated. Variation of the percentage increase in sugar concentration with hydrolysis at different pH, heating time and temperatures is shown in Table 3.
|
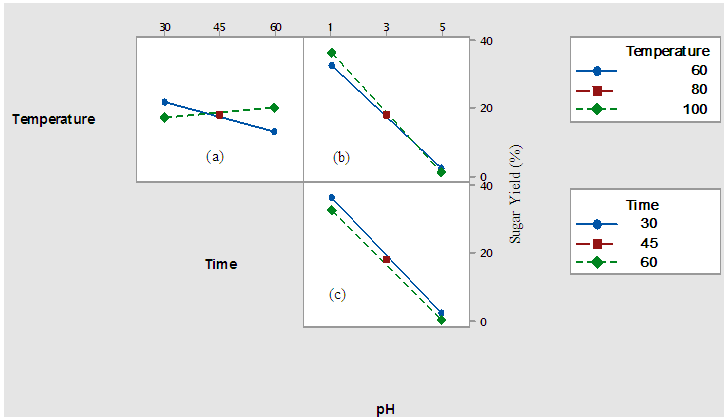 | Figure 1. Interaction Plots for Themal-acid hydrolysis of sugars from sisal boles juice |
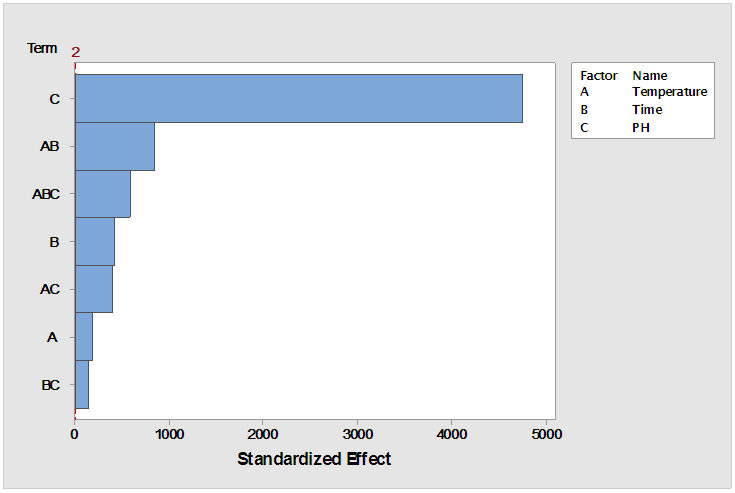 | Figure 2. Pareto chart for standardized effects (response is % Sugar, Alpha = 0.05) |
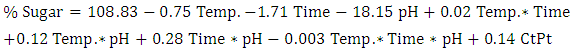 | (3) |
|
|
4. Conclusions
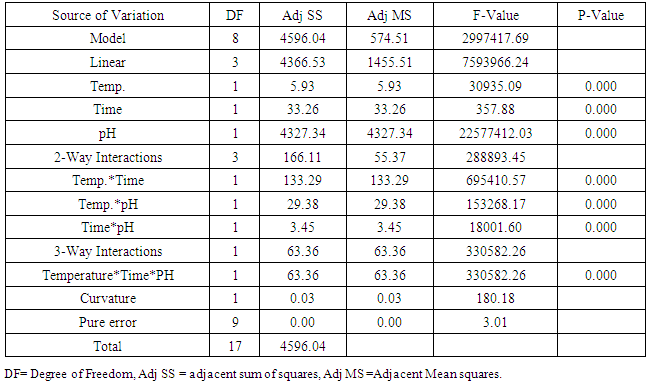
- Thermal-acid hydrolysis can be carried out at moderate temperature of 60°C with concentrated hydrochloric acid at pH of 1 within a period of 30 minutes to achieve a sugar yield of 39%. A combination of strong acid and increased temperature above 60°C appeared to have improved mass transfer effecting the hydrolysis followed by increased mobility of the hydrolysed sugars to the solution. Thus, the breakdown of complex sugars to simple sugars is basically a function of acid concentration (hydrogen ion concentration) and its distribution in solution. At lower juice pH therefore increase in hydrolysis was achieved as elaborated and analysed by the interaction plots. ANOVA analyses gave an evidence that linear, 2- way interactions and 3-way interactions of all the variables (temperature, time and pH) significantly affected the sugar yield at 95% confidence level with p-value =0.00. The hydrolysed juice was also subjected into fermentation for lactic acid and results showed favourable LA yield was obtained with the recommended thermal-acid hydrolysis in this study.
ACKNOWLEDGEMENTS
- We are grateful to Tanzania Commission for Science and Technology - COSTECH (Bioplastics Project-UDSM) and DAAD (In-Country scholarship) for financial support. Authors are also grateful to Mr. Jamal M. and Mr. Abdi A. for their support during data collection.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML