-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2018; 8(2): 36-40
doi:10.5923/j.chemistry.20180802.02

Characterization of Sisal Boles for Production of Polylactic Acid (PLA)
N. Msuya, J. H. Y. Katima, R. J. A. Minja, E. Masanja, A. K. Temu
Department of Chemical and Mining Engineering, University of Dar es Salaam, Dar es Salaam, Tanzania
Correspondence to: N. Msuya, Department of Chemical and Mining Engineering, University of Dar es Salaam, Dar es Salaam, Tanzania.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Biobased biodegradable plastics (bioplastics) show a large range of properties which can compete with non-biodegradable thermoplastics in packaging, textile and biomedical applications. Polylactic acid (PLA) is one of the most promising bioplastics, which are synthesized by polymerization of Lactic Acid (LA). Traditionally LA is produced from pure sugars and food crop sources like potatoes, cassava, corn, wheat, rice, sugar beet, sugar cane, and others. However, because of competition with existing uses, alternatives raw materials are being researched. One such alternative is the sisal boles. Sisal bole is part of the 98% of the sisal biomass which is traditionally counted as waste. Sisal boles as raw material for PLA are advantageous since it is not competing with food. This work characterizes sisal boles juice and its potential to produce biodegradable plastics. The sisal boles juice was extracted from chopped autoclaved boles using a hydraulic pressing machine. The juice was hydrolyzed to allow the sugar polymers to break into monomer sugar. The boles have been found to comprise of up to 97.94% (w/v) organic matters and total sugar content in juice of up to 30% (w/v). The produced juice mineral content levels were within the recommended working fermentation range with necessary nutrients for LA producing microorganisms. Sisal boles can therefore be one of the good raw materials for lactic acid production.
Keywords: Sisal Boles, Characterization, Lactic Acid Production, Biodegradable Plastics, PLA
Cite this paper: N. Msuya, J. H. Y. Katima, R. J. A. Minja, E. Masanja, A. K. Temu, Characterization of Sisal Boles for Production of Polylactic Acid (PLA), American Journal of Chemistry, Vol. 8 No. 2, 2018, pp. 36-40. doi: 10.5923/j.chemistry.20180802.02.
Article Outline
1. Introduction
- Tanzania has been earning foreign exchange through export of sisal products and in 2014 she was the second world's largest sisal producer (37,500 tonnes) after Brazil [1, 2]. Tanzania has a unique position to increase the sisal production as she has comparative and competitive advantages in terms of suitable weather, soil (soil surface temperatures often exceeding 55°C [3] and human capital which are essential catalysts to the growth of the industry. The Tanzanian government would like to ensure that the sisal subsector contributes to its agricultural sector policy objectives of improving food security; crop varieties; farming systems; production technologies and efficiencies, and crop diversification [4]. Sisal industry can improve food security to workers by assuring that they have economic access to sufficient, safe and nutritious food to meet their dietary needs and preferences for an active and healthy life. It is acknowledged that the resent revival of sisal industry in Tanzania is attributed to the smallholder farmers with 6 to 200 hectares. They contributed a third (12,500 tonnes) of the total sisal production in 2014 [1, 5]. In a typical sisal production process, a very small portion, about 2%, of sisal plant (sisal fibres) is used to produce twine, packaging, carpets, marts, thread, fine yarns, ropes and roofing tiles [6]. The remaining 98% biomass which include leaves decortications waste, wastewater and sisal postharvest waste (sisal poles, core-sisal boles and stubs of leaves that remain on the boles after every cutting) is discarded as waste [2, 7]. Generally, fibre is only 4% of leaf weight and production of one tonne of fibre generate waste amounting to 24 tonnes of leaf residues, 100 m3 of wastewater and 4.7 tonnes of sisal boles [8]. The liquid wastes end up in stream and other water bodies while the solid waste is normally disposed of by burning or dumping on land and leaving it to rot thereby generating greenhouse gases such as methane. It is an undisputed fact that the economical sustainability of the sisal industry, depends on utilization of this bulk waste material (98%). Although highly fibrous and poor in nitrogen, sisal waste has high contents of organic matter and rich in total sugars (boles), which makes it potentially suitable for production of a wide range of high value commodities [2, 9]. In efforts to increase sisal plant productivity, several studies have been undertaken to assess potential for production of other products: such as citric acid and ethanol [7, 9-11]. These researches demonstrate the viability of valorisation of sisal waste. One of the potential products that has less or not been studied is the Lactic Acid (LA) which is a raw material for production of bioplastics products (PLA). PLA is a sustainable, environmentally friendly, non petroleum dependent and low-carbon bioplastics for future applications [12, 13]. This study investigated the viability of transforming sisal waste (sisal boles) into Polylactic Acid (PLA) or Polylactide, which is a bio-plastic. This is biobased biodegradable thermoplastic aliphatic polyester or so called biodegradable plastic.
2. Material and Methods
2.1. Sisal Boles Preparation and Handling
- Samples of sisal boles from Agave (Hybrid 11648) plants of 10-12 years were randomly collected from Fatemi sisal estate, Morogoro and Katani Ltd, Tanga. Samples were collected from different sites to investigate if there is any impact on geographical location (weather and soil characteristics e.g. mineral content, fertility, etc., temperature and rainfall). Eight samples of boles were collected randomly from one-hectare farm area and transported to the laboratory. The boles were weighed before and after removing the leaf stubs. They were then cleaned using raw municipal water. The moisture contents for each bole were measured following the standard procedures [14]. The samples for moisture content measurements were taken from top, centre and bottom part of the boles. The values were expressed as percentage of the boles weight. The boles were then stored in a refrigerator set at 4°C to minimize microbial activities. The sisal plant, boles and initial preparations are shown in Figure 1.
 | Figure 1. Sisal boles preparation: A-Sisal plant; B: Sisal bole with stubs; C: Leaves stubs removal; D: Bole cleaned with tap water; E: Boles weighing |
2.2. Compositional Analysis of the Sisal Boles
- The sisal boles were then chopped using panga and knives into small pieces (3-5 cm) for compositional analysis. Organic carbon content was measured following the procedure by Walkeley- Black, [15]. The Nitrogen content (total kjeldahl) and crude fibres were determined using standard procedures [14]. Mineral contents (Ca, K, Na, Cu, Zn, Mn, Mg, Fe, and Co) were measured following the Standard Methods for the Examination of Water and Wastewater [16]. Dried, digested and well filtered sisal bole samples were analysed using Atomic Absorption Spectroscopy (Varian AA240) with graphite tube atomizer model GTA120, A hollow cathode lamp for each mineral element was selected and set to its proper wavelength (ηm) such as Ca2+(422.7), K+(766.5), Mg2+(285.2), Na+(589.0), Cu2+(324.8), Fe2+(248.3), Mn+(279.5), Zn2+(213.9) and Co+(240.7). The Standard Methods for the Examination of Water and Wastewater [16] were used to determine the COD, the total solids (TS) and volatile solids (VS).
2.3. Compositional Analysis of Sisal Juice
- Juice extraction process involved chopping of sisal bole into small pieces (about 5-15 mm3) to increase the surface area during pressing. The juice was extracted from 1 kg sample of chopped boles using hydraulic pressing machine operated by hydraulic jack with a capacity of 16 tonnes. The machine extraction housing is made of stainless steel housing. Three alternatives were tried to maximize juice extraction. The first option involved direct pressing sisal boles choppings without heating, the second involved heating them at higher temperature above 100°C this was done by autoclaving at 121°C for 5 minutes. The autoclaved chopped bole parts were then cooled to room temperature (30°C±3) before pressing. The third option involved mixing the boles with 1L of boiling water (100°C) before pressing to increase solubility of soluble components in bole mass to bring about the easiness during extraction process. The biomass residue after the first juice collection in either option was tested for sugar concentrations. This was done by adding half a litre of hot water to enhance wet blending of the biomass residue, and pressed to extract the juice to be tested. The process was repeated until maximum juice (sugar) extraction was obtained. Each batch of juices was subjected to physical-chemical characterizations and average values were used and reported. The extracted juice was hydrolyzed to allow the sugar polymers to break into monomer sugar before analysis. The hydrolysis method by Busairi, [17] was adapted. The method involved transferring 50 ml of the juice into volumetric flask (100 ml) followed by addition of 5 ml of 2M HCl, boiled for 5 minutes and left to cool to room temperatures (27±2°C). The product was neutralized using 2.5M NaOH and then the solution made up to 100 ml by addition of distilled water [17]. Calibration curve (CC) for sugar determination was drawn using glucose standard sugars supplied by Sigma Aldrich and the slope was used during total sugar determination. The hydrolysates determination of total sugar was done using 3,5-Dinitrosalicylic Acid Method [18] using the calibrated curve already drawn. The absorbance of the solution was measured by using digital UV/VIS spectrophotometer (Labtronics LT-31) at 540 nm and used to obtain the sugar concentrations. The total sugar concentrations were calculated using equation (1) taking into account the dilution done during absorbance reading.
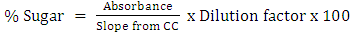 | (1) |
3. Results and Discussion
3.1. Sisal Boles Preparation, Handling and Juice Extractions
- The average weights of sisal boles before removal of leave stubs were 43.5±2.0 kg (Morogoro) and 57.3±1.5 kg (Tanga), and they were 11.55±1.0 kg (Morogoro) and 22.8±1.1 kg (Tanga) after the removal of leaves stubs. These shows that leave stubs cover more than 60% of whole sisal boles. It was important to remove the leave stubs from the core boles to avoid interference of chlorophly with juice quality. The average moisture contents for boles from Morogoro and Tanga were 72.15±2.0% and 60.72±1.8% respectively which matches well with those of Muthangya, [21], who found that the moisture content was between 60.29±1.1 to 76.78±1.0 percent. Although the moisture contents of the boles from Morogoro is higher, their core boles size is smaller than that of Tanga. The difference in moisture content may be attributed to difference in soils fertility, rainfall pattern, harvesting season of the year and age of sisal plant. Samples from Tanga were harvested during dry season.The study also found that the boles from Tanga comprises moisture content of 65.68±2.7 (%w/w), 57.04±1.9 (%w/w) and 52.68±3.5 (%w/w) corresponding to the top, centre and bottom section of the sisal bole respectively. The higher amount of moisture at the top section can be attributed to fact that, as the plant grows, the cells become matured upwards as new cells are generated. Young plant cells have more water content than earlier cells. Juice extraction results were 567±2.9, 791±0.6 and 653±2.9 volumes of juice per kg of sisal bole for chopped and pressed, autoclaved and mixed with boiling water, respectively. Thus, the autoclaved sisal boles gave the highest volume of juice compared to other methods. This can be attributed to higher temperature (121°C) used under autoclaving pressure of 1.5 bar, which led to rapture of sisal boles providing more surface area for juice extraction.
3.2. Compositional Analysis
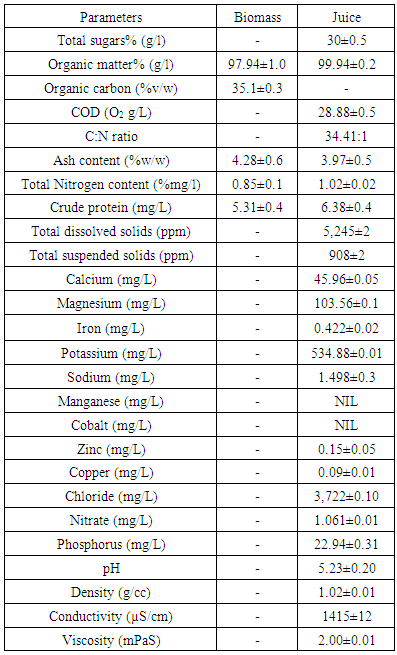
- The calibration curve for the standard sugar gave a linear relationship with R2 of 0.9971. Boles from Morogoro gave a total sugar of between 8-15% (m/v) which is similar to what was obtained by Elisante & Msemwa, [9] who used samples from Morogoro. Boles from Tanga produced 10-30% (m/v) total sugars which are similar to what was obtained by Ngonyani, [7] who used samples from Tanga. The differences in amount of sugars for the samples from the two sites, Morogoro and Tanga, might be due age of sisal plant used, soil characteristics and the geographical locations which need to be further researched. Tanga has the largest sisal plantation in Tanzania with more than five estates with land area 3,635 hectares. Table 1 gives the compositional analysis of the boles and the juice extracted from these boles.
|
4. Conclusions
- Sisal boles are available in Tanzania in plentiful amount, it is estimated that 176,250 tonnes of sisal boles can be produced per year. About 1.6 million tonnes of sugars can be produced from the sisal boles and when fermented can produce 16 million kg of LA. LA can be polymerized to PLA which has a huge market potential for packaging material especial short life food packaging. This study has shown that, sisal boles which have been traditionally treated as waste can be utilized to produce products with significant positive environmental impacts. Production of Lactic acid from sisal boles and PLA from LA produced is discussed in other papers [6, 22]. The produced sisal boles juice had sugar concentration of 30% which is higher than the recommended juice fermentation range of 8-15% (w/v). The produced juice mineral content levels were within the recommended working fermentation range with necessary nutrients for microorganisms. Sisal boles can therefore be one of the good raw materials for lactic acid production.
ACKNOWLEDGEMENTS
- We are grateful for financial support of this work to COSTECH (Bioplastic Project-University of Da es Salaam) and DAAD (In- Country scholarships). Special thanks to Mr. Ashraf Abdi (Research Assistant-Bioplastic Project) for his technical support and Mr. Mfuruki Jamal for his support during data collection.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML