-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2018; 8(1): 13-18
doi:10.5923/j.chemistry.20180801.03

Separation and Purification of Lactic Acid from Sisal Wastes
N. Msuya, R. J. A. Minja, J. H. Y. Katima, E. Masanja, A. K. Temu
Department of Chemical and Mining Engineering, University of Dar es Salaam, Dar es Salaam, Tanzania
Correspondence to: N. Msuya, Department of Chemical and Mining Engineering, University of Dar es Salaam, Dar es Salaam, Tanzania.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

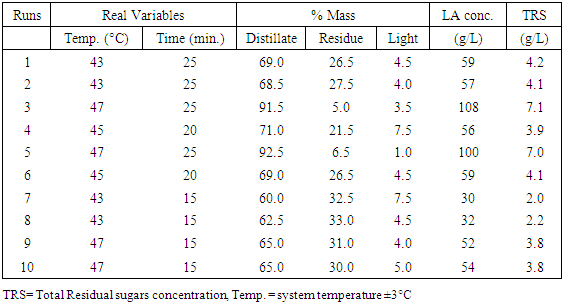
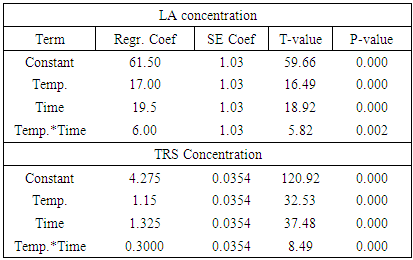
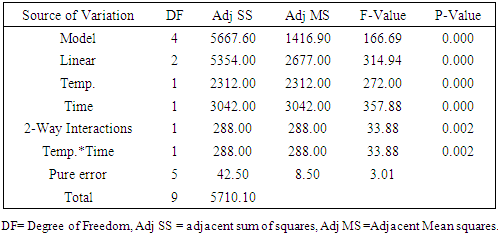
The aim of this paper was separation and purification of Lactic acid (LA) produced from Sisal Waste by investigating the influence of modified hybrid short path evaporation (M-HSPE) system on the concentration of LA. A 22 full factorial design was used to study the influence of temperature and time on the concentration of LA and % mass obtained in distillate and residue flask. Minitab V.17 was used in designing the LA concentration experiments and in calculating the effect of each variable and their interactions. Higher LA concentration of 108 g/L occurred at system temperature of 47°C for 25 minutes. This represented a concentration of 4.5 times the initial concentration (24 g/L) with 100% recovery in residues stream/ flask. Therefore, the M-HSPE system was able to concentrate LA to almost twice the original hybrid short path evaporation (HSPE) system. According to ANOVA, LA concentration presented a correlation coefficient of 0.993, which confirm that, linear model correlated well with the experimental data. The system temperature (Temp.), operation time (time) and the interaction between system temperature and time were statistically significant variables for LA concentration at alpha = 0.05.
Keywords: Lactic acid separation, Sisal boles juice, Modified hybrid short path
Cite this paper: N. Msuya, R. J. A. Minja, J. H. Y. Katima, E. Masanja, A. K. Temu, Separation and Purification of Lactic Acid from Sisal Wastes, American Journal of Chemistry, Vol. 8 No. 1, 2018, pp. 13-18. doi: 10.5923/j.chemistry.20180801.03.
Article Outline
1. Introduction
- Lactic acid (LA) can be produced through chemical synthesis or fermentation route and is used in food and chemical industries. In Fermentation route, microorganisms eat sugars containing materials like sisal boles and excrete LA as product. Sisal bole is a stem part of a sisal plant (Agave). It is reported that in Tanzania, only 2% of sisal plant (sisal fibres) is used to produce fibres [1]. The remaining 98% biomass (sisal poles, sisal boles and stubs of leaves that remain on the boles after every cutting) is discarded as waste [2, 3]. Sisal boles waste contain 26-30%(w/v) sugars inform of inulin [4], that could be fermented to produce LA. After fermentation, the LA fermentation liquor contains LA, together with various impurities such as unreacted raw materials (e.g. sugars), microbial cells and culture media, i.e. derived saccharides, amino acids, carboxylic acids, proteins and inorganic salts [5-9]. Therefore, the separation and purification of LA from other impurities is mandatory to obtain the quality needed in the polymers synthesis [9, 10]. Purification and separation of LA after fermentation involves removal of biomass and other solids from the broth, acidification of the fermented product with strong acids to liberate the LA, removal of inorganic salts and concentration of LA [5]. However, the procedure for LA purification is rather difficult due to its chemical behaviour, as it shows strong affinity to water and has low volatility [7]. The presence of two adjacent functional groups (acid and alcohol) in a small molecule of LA gives it, its high reactivity with all bases, as well as its tendency to decompose at high temperatures (227°C at atmospheric pressure) [6, 8]. Purification of LA from fermentation broth has been studied using different techniques, including separation with membranes such as nanofiltration and electrodialysis [11-13], adsorption or ion exchange [14, 15], reactive distillation [16], [17] and hybrid short path evaporation [7, 8, 16]. It is reported that, membrane technologies are very potential and have particular advantage of simultaneous separation and concentration of LA [18]. However, membrane separation performance is compromised by fouling which requires frequent cleaning of the dialyzer [11, 18]. The cost of equipment can be relatively high and the method requires high energy input [18] as such, it was not used in this research as the main separation technique.On one hand, the major disadvantage of the adsorption or ion exchange method is that it requires regeneration of ion exchange resin and adjustment of feed pH to increase the sorption efficiency thus, requiring large amount of chemicals [8, 9, 14]. On the other hand, distillation requires low vacuum that forces higher molecular weight component such as sugar and protein to leave the system as residue. The formation of oligomers (high boiling esters and dimmers) limits an overall distillation yield. HSPE was a preferred alternative method for recovery and concentration of LA [8], [19] as it employs lower temperatures of operation (60-80°C), due to application of vacuum, and hence requires short residence time [7]. This method also reduces the tendency of LA decomposition and it is energy efficient [6, 20]. The description of the method and important parameters to evaluate its performance are described by Komesu et al., [8]. The aim of this paper was separation and purification of LA produced from Sisal Waste by investigating the influence of modified hybrid short path evaporation (M-HSPE) system on the concentration of LA.
2. Material and Methods
2.1. Feed Preparation
- The raw material used was fermented broth from sisal boles juice. Boles were chopped and pressed using hydraulic pressing machine operated by hydraulic jack to extracted juice which was then hydrolyzed to allow the sugar polymers to break into monomer sugar before fermentation. The hydrolysis method by Busairi, [21] was adapted. The microorganism strain utilised was Lactobacillus delbrueckii WLP677 from the White Labs Inc. (CA 92126 USA). The bacterial culture was grown in 50 ml of De Man, Rogosa and Sharpe agar (MRS) medium in 250 ml Erlenmeyer flask [22]. After sterilization at 121°C for 15 minutes using portable steam Autoclave (Heuer, 220V and 50 Hz) and cooling to room temperature (30°C ± 2), the medium was inoculated with 10% inoculum level. The medium was then incubated at 37°C for 24 hrs under stationary conditions for microbial growth before fermentation. The pH of the fermentation medium was regulated before fermentation to 5 or 6, using 10M sodium hydroxide. The broth was then separated after 72 hrs fermentation. The separation of cells and other insoluble proteins was done by centrifugation at 5000rpm for 15 minutes. The broth was then clarified using ultrafiltration system (Amicon 8400). The system used an inorganic membrane with a molecular weight cut-off (MWCO) of 5kDa (Microdyn-Nadir UP005) to remove micro-particles. The clarified broth was acidified to pH that is less than pKa of LA (3.86) with strong acids (H2SO4 or HCl) to liberate the LA from the sodium lactate formed when sodium hydroxide was used to control fermentation pH. The liberated LA was then subjected to ion exchange resin for more purification.
2.2. Multivalent Removal
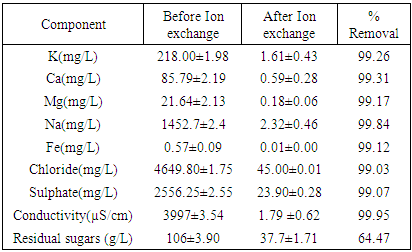
- The clarified fermentation broth was then passed through ion exchange resins in order to remove metal ions like potassium, sodium, magnesium and calcium. The experiment was performed in bulk per batch using a glass column of 3.4 cm diameter and 65 cm length, where 450 g of an ion exchange resin was placed. Only 75% of the column was packed with resins to provide room for expansion of the resins bed. The anion exchanger used was Indion FFIP (Ion Exchange India Ltd), a weak basic anion exchange resin which contains only tertiary amino acid groups. The cation exchanger resin was Indion 225H (Ion Exchange India Ltd), a strongly acidic macro porous cation-exchange resin with uniform size beads. The ion exchange resin system, Figure 1, operated simultaneously, that is the feed was first introduced to a cation exchange resin column (1) at the bottom and left the column at the top where it was connected to the anion exchange resin column (2). The broth was fed to the anion exchange resin column at the bottom and left the system at the top of the column to the product reservoir. A peristaltic pump Skan-AG (Watson Marlow Ltd) was used to pump the feed. The experiment was concluded when the processed volume was high enough to saturate the column. The regeneration of the resin was conducted from the column top to the bottom to reduce the consumption of reagents. Backwashing was done using distilled water for 45min at a rate of 1 mL/s before regeneration. The regeneration experiment was done at room temperature using 1.1M HCl and 0.75M NaOH for cation and anion exchanger resin, respectively.
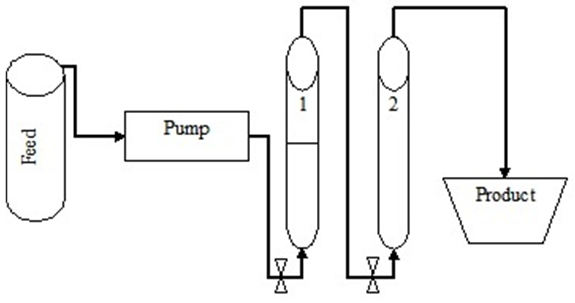 | Figure 1. Ion exchange resin system |
2.3. LA Concentration by M-HSPE
- After the removal of ions, cells and other unreacted materials like protein, the LA was concentrated by removing water and other acids like acetic acid. The HSPE system by Komesu et al., [8] that depends on the difference in boiling temperatures (volatility) was adopted as modified hybrid short path evaporation (M-HSPE) system. The LA fermented broth was manually fed into a round bottomed necked flask connected to the system in batches of 200 ml, Figure 2. The system allowed the heated flask with feed connected to another flask (distillate) using the fractionating column to separate and return the dense volatiles back to the flask and the less dense escaped to distillate flask. Digital stirring heating mantle (DSHM 2000 – Azzota Cooperation) with temperature control was used to control the system temperature and provide uniform mixing using magnetic stirrer.
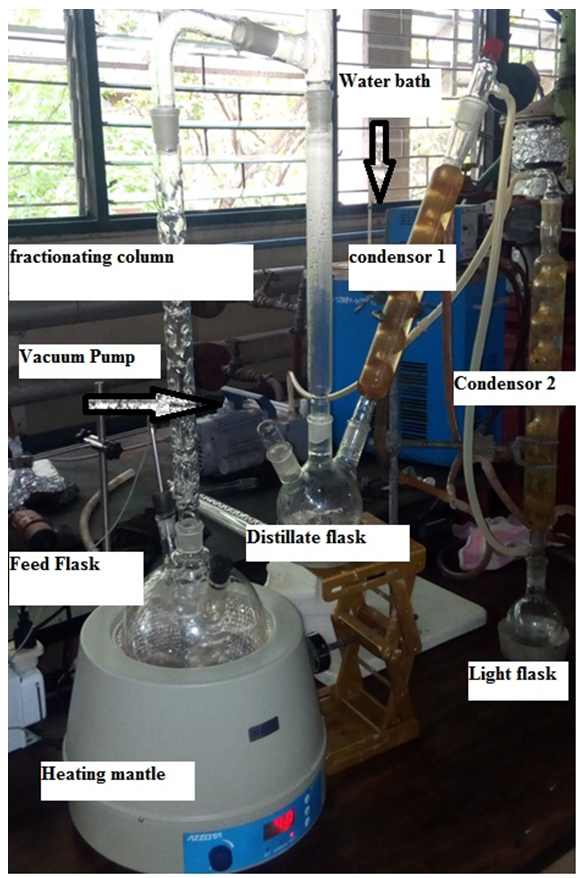 | Figure 2. Modified Hybrid Short Path Evaporation System |
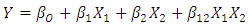 | (1) |
2.4. Analytical Methods
- The pH and conductivity of the sample were measured using pH meter (CH-9100 Metrohm) and conductivity meter (B36356 Thermo Scientific), respectively. The amount of metal ions: Potassium, sodium, magnesium and calcium were measured using Atomic Absorption Spectrometer (Varian AA240). Sulphate content was analysed using colometric method [23] to determine total sulphate. Chloride content of the LA was analysed by potentiometric titration as described by APHA, [23]. LA concentration was measured using UV-IV digital spectrophotometer (Labtronics LT-31) following the method by Borshchevskaya et al., [24]. The method was selected since it is relatively cheap and has an error of less than 3% compared to HPLC method. The efficiency of the system on ionic and cationic removal and LA concentration was calculated using the concentration before and after passing the broth in the system. The lactic acid recovery rate was calculated using Equation 2 as per Komesu et al., [8].
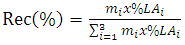 | (2) |
3. Results and Discussion
- By means of centrifugation and ultrafiltration the proteins and cells were removed and through ultrafiltration system colour causing compounds like pigments from sisal juice and agar used were removed as the broth was changed from yellow to colourless. The feed to the ion exchange system contained mainly LA, water and inorganic salts. The main cations were sodium, calcium, potassium and magnesium while the main anions were chloride and sulphate. High concentration of Na is due to use of NaOH during fermentation pH regulation. The use of conc. HCL and/or H2SO4 to reduce sample pH to 1 during hydrolysis increase concentration of chloride and sulphate in the product as shown in Table 1. The ionic composition of the fermented broth before and after ion exchange is given in Table 1. The percentage removal was calculated using equation 3:
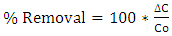 | (3) |
|
|
|
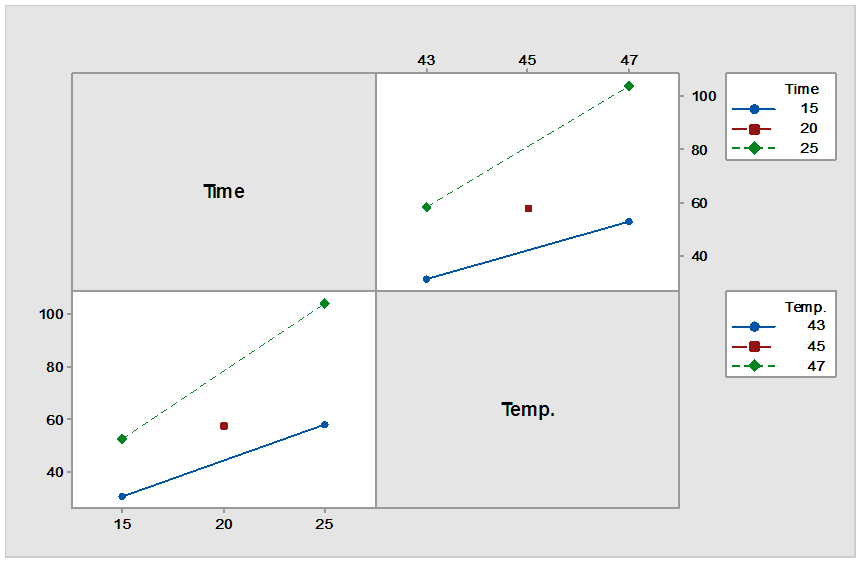 | Figure 3. Interaction plot for LA concentration |
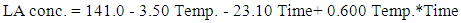 | (4) |
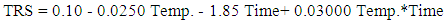 | (5) |
|
4. Conclusions
- The clarified fermentation broth used as feed in the ion exchange system in this work contained mainly LA, water and inorganic salts. The main cations were sodium, calcium, potassium and magnesium while the main anions were chloride and sulphate. By means of centrifugation and ultrafiltration the proteins cells and more than 60% of the residual sugars were removed. Ultrafiltration also removed the colour causing compounds like pigments from sisal juice and agar. The separation system was able to remove ions by 99% on dry basis for both cations (K, Mg, Fe, Ca and Na) and anions (Chloride and sulphate). All the LA was found to remain in the residue (feed flask) stream. The distillate stream and the light stream were found to have no LA which means therefore, the temperatures ranges used were sufficiently low to condense LA to remain in the feed flask. It was possible to remove more volatiles from residue flask to distillate up to more than 90%. Higher LA concentration of 108 g/L was obtained at a system temperature of 47°C for 25 minutes. This represented LA concentration of 4.5 times the initial LA concentration (24 g/L) before the purification process with 100% recovery in residues stream/ flask. Therefore the M-HSPE system was able to concentrate LA to almost twice the original HSPE.According to ANOVA, LA concentration presented a correlation coefficient of 0.993, which confirm that, linear model correlated well with the experimental data. The system temperature (Temp.), operation time (time) and the interaction between system temperature and time were statistically significant variables for LA concentration at alpha = 0.05.
ACKNOWLEDGEMENTS
- We are gratefully for the financial support by Tanzania commission for Science and Technology (COSTECH) under Bioplastics Project and DAAD- in Country scholarship through University of Dar es Salaam (UDSM)-Tanzania. Authors are also grateful for technical support by Mr. Ashraf Abdi, Research assistant, Bioplastics project (UDSM).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML