-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2017; 7(5): 171-175
doi:10.5923/j.chemistry.20170705.03

Writing Electron Configuration: An Algorithmic Approach
Shahrokh Ghaffari, Shadi Abu-Baker
Chemistry Department, Ohio University Zanesville, Zanesville, United States
Correspondence to: Shahrokh Ghaffari, Chemistry Department, Ohio University Zanesville, Zanesville, United States.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The goal of the method presented in this paper is introducing students to a simple procedure to arrive at electron configuration and discover its relevance to the Periodic Table. Once that is accomplished, more complicated configuration can be introduced. Writing an electron configuration or electron diagram is an essential topic covered in all introductory chemistry courses. This requirement often proves challenging for certain students. This procedure employs the periodic table as the first step in a multi-step approach that progressively advances students toward the development of an electron diagram. This method is designed specifically for students with little to no background in chemistry. This method can be used both as lecture supplement or a laboratory experiment.
Keywords: Electron Diagram, General Chemistry, Laboratory Experiment, Alogrithms
Cite this paper: Shahrokh Ghaffari, Shadi Abu-Baker, Writing Electron Configuration: An Algorithmic Approach, American Journal of Chemistry, Vol. 7 No. 5, 2017, pp. 171-175. doi: 10.5923/j.chemistry.20170705.03.
1. Introduction
- To demonstrate the electron configuration of the ground state of atoms, a number of methods, diagrams, and analogies have been presented [1-12]. Although, there are exceptions in the arrangement of electrons in different energy and sub energy levels, in most cases this can be explained by a simple rule. In most cases, students memorize one of the different versions of the Aufbau Principle of the distribution of electrons in different orbitals following Hund’s Rule and the Pauli Exclusion Principle. Using quantum mechanics to calculate values of various quantum numbers to give the electron distribution of an atom is challenging and beyond the grasp of students with little or no chemistry background.
2. Step I
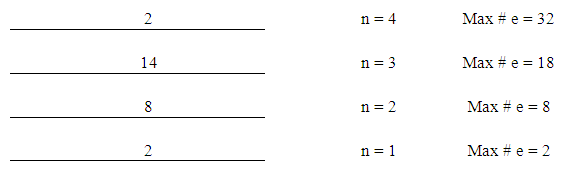
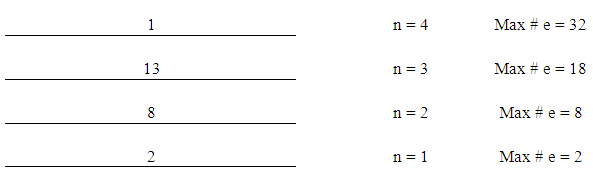
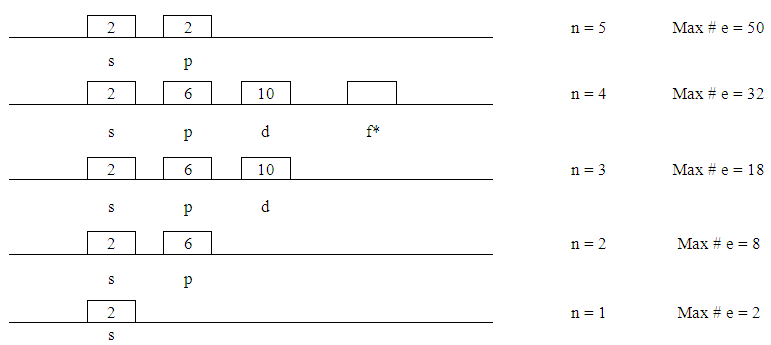
- This step consists of five parts;1. Figure out the number of energy levels that have electronsThe number of energy levels = row numberFor example, since all elements from K (potassium) to Kr (Krypton) are in row number 4, they all have 4 energy levels. Write down the maximum possible number of electrons for each energy level using “2xn2”.
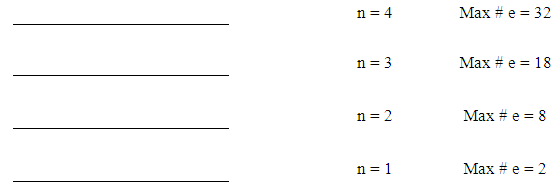 | Figure 1. Energy Levels with Maximum Number of Electrons |
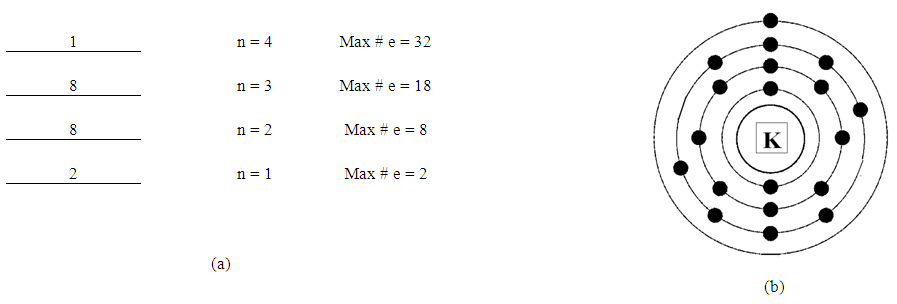 | Figure 2. Potassium, K (19) |
 | Figure 3. Iron, Fe (26) |
 | Figure 4. Chromium, Cr (24) |
3. Step II
- 1. The next level boxes are added to each energy level with a maximum number of electrons going in each one. The number in each box indicates the possible maximum number of electrons in each. To assign the possible number of electrons in each box start with “2” in the first box on the left. For the next box “4” is added (2 + 4 = 6). The plus “4” rule is followed to assigned the number of possible electrons in the rest of the boxes on the same energy level.
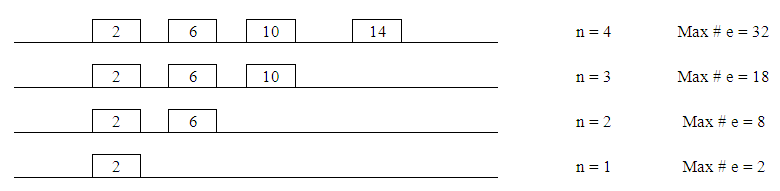 | Figure 5. Total Electron Distribution in Orbitals |
 | Figure 6. Labeling Orbitals |
 | Figure 7. Manganese, Mn (25) |
 | Figure 8. Tin, Sn (50) |
4. Step III
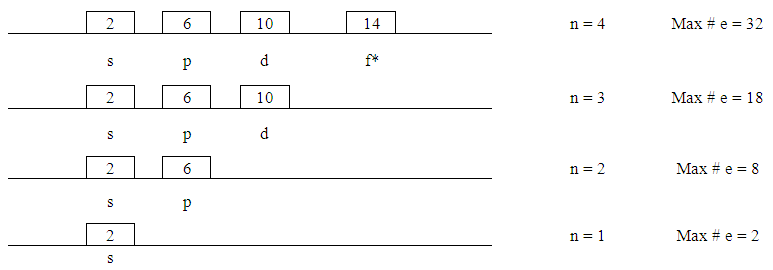
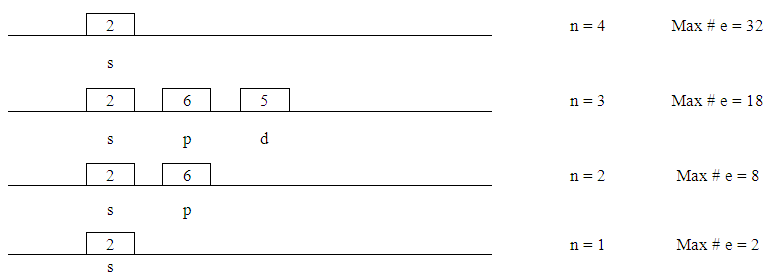
- 1. At this point the idea of sub-boxes is introduced that each can hold not more than two electrons. Starting with “s” there is only one orbital. As we move to the next “p” increases by +2-- that is 3 orbitals--and for “d” there are 2 more and so on.
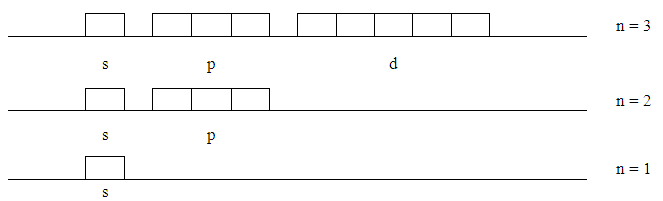 | Figure 9. Splitting Orbitals |
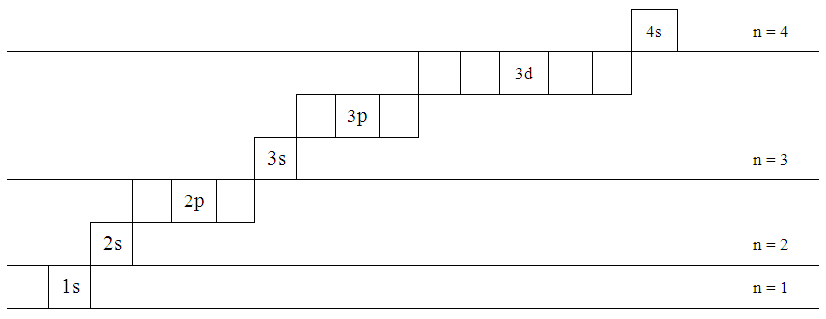 | Figure 10. Orbital Energy Level |
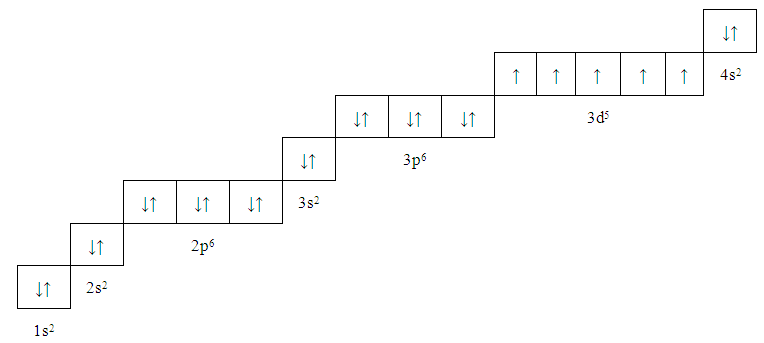 | Figure 11. Manganese, Mn (25) |
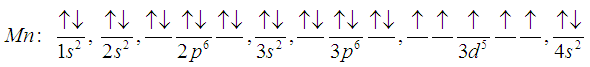 | Figure 12. Manganese, Mn (25) |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML