-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2017; 7(4): 145-151
doi:10.5923/j.chemistry.20170704.03

Comparative Analysis of Leachable Heavy Metals in Earthenware Clay Deposits in the Central and Volta Regions of Ghana
Victus Bobonkey Samlafo
Department of Chemistry Education, University of Education, Winneba, Ghana
Correspondence to: Victus Bobonkey Samlafo , Department of Chemistry Education, University of Education, Winneba, Ghana.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This paper, sought to compare and contrast the potential leachable heavy metals in earthenware clay deposits in the Central and Volta regions of Ghana, using the Atomic Absorption Spectrophotometer (AAS). The study also tried to establish the suitability of which clay deposit is the ideal raw material for earthenware products used as food wares, based on toxic heavy metal and micro nutrient/essential metal levels. The toxic metals determined were Pb, As, Hg, and Cd, while the micronutrients/essential elements examined were Cr, Zn, Mn, Cu, and Fe. The results showed that, apart from Hg and Cr, there was no statistical difference in heavy metal levels in the two regions. Earthenware clay deposits in the two regions were found to be suitable raw materials for food ware products based on their heavy metal levels. The reproducibility of the analytical method was assessed by analysis of the standard reference material IAEA soil-7. The values obtained, compared favourably well with the recommended values as Spearman correlation coefficient was +0.96%. The experimental values were within ± 4% of the recommended values. The measurement precision specified by the relative standard deviation was within ± 5%. The error margins are standard deviations. A two -tailed student’s t-test was used to establish any statistical differences between the mean concentrations of the two earthenware clay deposits. The level of probability at which significant differences existed between the deposits was set at p < 0.05 at 95% confidence level. In general, the two clay deposits were found to be suitable sources of raw materials for food ware products.
Keywords: Heavy metals, Essential elements, Clay, Earthenware, Central region, Volta region
Cite this paper: Victus Bobonkey Samlafo , Comparative Analysis of Leachable Heavy Metals in Earthenware Clay Deposits in the Central and Volta Regions of Ghana, American Journal of Chemistry, Vol. 7 No. 4, 2017, pp. 145-151. doi: 10.5923/j.chemistry.20170704.03.
Article Outline
1. Introduction
- Clay consists of a large number of tiny flat plates that are stacked together with a thin layer of water separating each crosslink. Alumina (Al2O3) and silica (SiO2) combines with water and other elements in various proportions to form clay minerals. Heating clay at high temperature withdraws this water resulting in the formation of bonds between the plates, holding them in place and forming a hard solid [1]. Clay is one of the cheapest and most easily available raw materials. The difference in the texture, colour, and quality of clay depends on how it was deposited and the type of mineral it collected during its formation [1]. Clays, in general, are used for a variety of purposes. These include its use as raw material for the ceramics, refractory and cement industries, as filling material in the pulp and paper, toothpaste and paint industries as well as for production of aluminum sulphate (alum), among others. A particular application depends on the physical, chemical and mineralogical characteristics of the clay [2]. The origin of pottery and its uses have been the subject of much research. Pottery was used for cooking, storing, processing, preserving, serving, and transporting food, as well as, for ritual purposes [1].The potters have designed the pot in such a way that, the narrow mouth helps to prevent water spillage. This ergonomically created design is still sustainable in water storage and transport methods. Earthen pots have pores. When water is poured into the pot, a small amount of it exits through these pores and evaporates from the surface of the pot, thus making the pot (and remaining water) cooler than before and hence does not require electricity to cool water stored in it. Another benefit of the clay pot is the alkaline nature of clay. The alkaline clay interacts with the acidity of water and provides a pH balance. Water stored in an earthen pot is therefore gentle on the throat and ideal for people suffering from a cough and cold [3]. In this virtual world, where everybody is abreast with technology and advancement, there are people who still rely on traditional pot for purifying, cooking and storing water. This makes earthen pots sustainable. It has values and beliefs attached to its forms and functions. Moreover, the material decomposes back to nature without polluting the environment. Thus earthen pots are feasible to the masses who cannot afford expensive water purifiers [3]. Clay products have been in use from time immemorial as food wares. For some time, it has been thought that the constant use of clay items as food ware for different domestic purposes could be a source of health hazards. This is due to the leaching of heavy metals from their surfaces into food and beverages cooked or stored therein under different conditions such as pH, type of food, high temperatures of cooking and contact time of cooking [4]. The same study reported 1.092 mg/L, 0.196 mg/L and 57.99 mg/L of Pb. Cd, and Fe leachates respectively, in banana liquor (pH 4.7) as alcoholic beverage prepared in the traditional pot in Rwanda [4]. Apart from mineral elements, clay serves as a reservoir of chemical and biological agents. Among the chemical agents are heavy metals, radioactive gases and organic chemicals [5].Heavy metals such as Fe, Mn, Cu, Zn, Co, Cr, V, Ti, Cd, Hg, Mo, As, Se etc occur naturally in clay/soil. However, the concentrations of these elements are frequently elevated because of contamination. The sources of contamination include agriculture, domestic and industrial pollution. Minerals such as Fe, Cu, Zn, and Mn are essential nutrients and play important roles in biological systems. However, the same mineral elements can produce toxic effects at high concentrations. Meanwhile, Hg, As, Cd, and Pb are toxic metals even in trace amounts [5]. Also, the Russian sanitary hygienic GOST 17.4.102-83 classified As, Cd, Hg, Se, and Pb as highly hazardous elements.This list of general toxicity is also applied in assessing the hazard of metals/metalloids in soils despite the fact that it ignores the interaction between the pollutants and soil components, which leads to misinterpretation of their toxicity [6].Lead, for example, is considered to be among the most dangerous metals for human health because it affects the central nervous system, causes anemia and gastrointestinal damage, and is associated with alterations in genetic expression. Cadmium is even more dangerous, being 10 times more toxic than lead, and is an element to which humans are readily exposed due to its large industrial uses [7]. A study conducted on geophagic clayey soil sold in three major markets (Madina, Makola, and Ashiaman) in Ghana, revealed higher levels of As, Pb, Hg Cd and Co. These results were higher than the WHO/FAO requirement and levels established by US Department of Agriculture, the study also established the fact that, these harmful elements exist in clay deposits [8].This paper sought to compare and contrast the heavy metal levels in earthenware clay deposits in the Volta and Central regions of Ghana and assessed their suitability as raw materials for earthenware products used as food wares.
2. Experimental
2.1. Study Areas
- Vume is the study area in the South Tongu District of the Volta region of Ghana and has Sogakope as its capital. The South Tongu District lies between latitudes 6°10’ and 5°45’ North and longitudes 30°30’ and 0°45’ East. The district is generally low lying by virtue of its location within the coastal savannah plain, with characteristic coastal savannah vegetation but rises gradually to a height of 75 metres above sea level [9]. Numerous creeks and lagoons run parallel to the Volta River through the district, which serves as good breeding grounds for tilapia, shrimps and mud fish. The district lies within the wet semi-equatorial and dry equatorial climate zones. The northern part of the district lies within the wet semi-equatorial zone while the southern part is in the dry equatorial climatic zone. The climate of the district is also influenced by the southwest monsoon winds twice in a year resulting in a double maxima rainfall regimes in May-June for the major season and September – November for the minor season with an average of 195 mm and 73mm of rainfall respectively, with temperatures ranging between 22.6°C and 29.3°C [9].The underlying rocks in the district are metamorphic in origin. The major soils formed over these geological formations include Ziwai-Zebe complex, Tondo-Motawme complex and Agawtaw-Kpejeglo complex soils which are formed over the Dahomeyan acidic gneiss rocks. The district has both alluvial, gneiss and schists deposits as their parent rocks. The district is endowed with large clay deposits at Lolito, Vume and Sokpoe communities which are predicted by geologists to last for over 100 years if it is mined commercially and in a sustainable way.Most females are found to be engaged in earthenware craft and related trades than their male counterparts. On the other hand, a higher percentage of males undertakes skilled agriculture, forestry, and fishery than their female counterparts in the district [9].Mankessim, the second study area is located within the Mfantsiman district with Saltpong as its district capital in the Central region. The Mfantsiman district lies between latitude 5° to 5°20’ north and longitude 0°44’ to 1°11’ west. The district is low lying with loose quaternary sands along the coast and is characterized by undulating coastal dense scrub and grassland with isolated marshy areas [10].Mfantsiman is about 60 metres above sea level and drained by a number of rivers and streams including the Nkasaku, which empties into the Atufa lagoon in Saltpond and Aworaba which drains into Etsi lagoon in Kormantse.The municipality is endowed with rich natural resources including talc, granite, silica, and kaolin of commercial grade which is used in building construction and the ceramics industry. The vegetation consists of dense scrub tangle and grass, which grow to an average height of about 4.5m.Mfantsiman has an average temperature of 24°C and relative humidity of about 70%, with double maximum rainfall with peaks in May-June and October [10]. Petroleum and natural gas (not yet exploited) are also found on the continental shelf offshore of Saltpond. Other natural resources which are yet to be exploited include beryl at Saltpond and the areas between Winneba and Mankessim, feldspar at Biriwa and Moree, spondumene (lithium) at Saltpond, uranium at Abandze, columbite, and tantalite at the coastal belt between Cape Coast and Saltpond [10].
2.2. Sampling
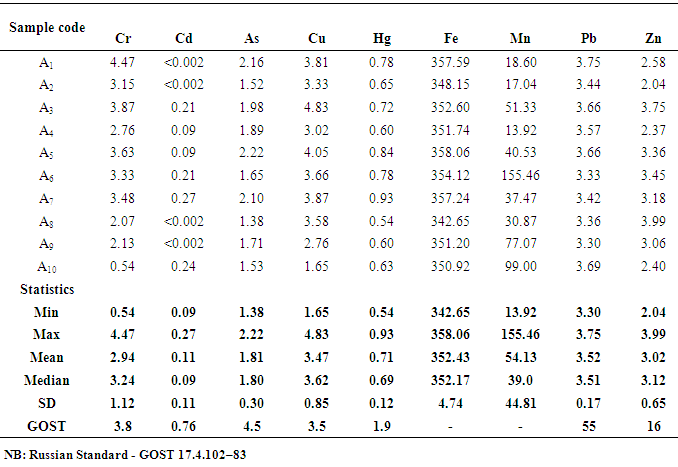
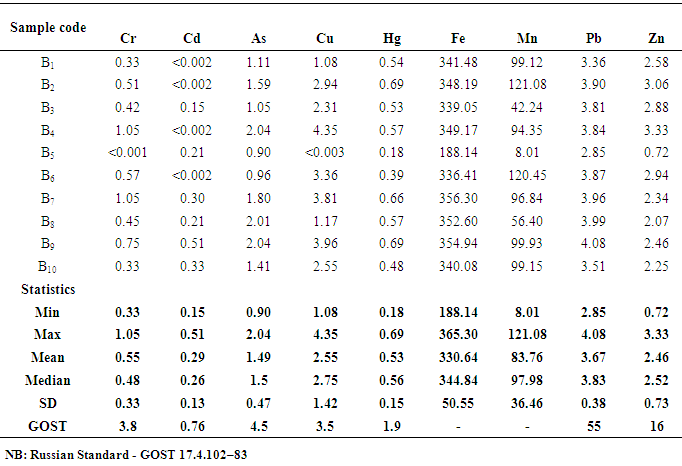
- The sampling procedure adopted provided for the lateral as well as the vertical variations in the physical and chemical properties of the clays. Samples (10 each) were collected three times from the Volta (Vume) and Central (Mankessim) regions of Ghana in 2015 using the Auger to a depth of 30 cm. The samples from the Volta region were labelled Bx and the Central region samples were labelled Ax where x=1-10. The samples were disaggregated, dried in an oven for 3 hours.The samples were then sieved using <40 mesh, homogenized and packed in polyethylene bags and stored in the laboratory until analysis. Five replicate samples were prepared for each sample and labelled. Two gram of each clay sample was weighed (five replicates) into 100mL polytetrafluoroethylene Teflon bombs. About 10 mL of concentrated HNO3 was added to each clay sample and allowed to stand for 10 minutes. About 30% H2O2 was also added to the mixture until the mixture no longer effervesced on the addition of H2O2. To each mixture in the Teflon bombs, 2 mL of concentrated H2SO4, and then 5 mL of concentrated HClO4 were added successively.The resulting mixtures were digested for 25 minutes in a Milestone microwave oven (Ethos 900) using the following operating parameters; 250W for 2 min, 0 W for 2 min, 250W for 6min, 400W for 5 min, 650W for 5 min and 5 min for venting [11]. The rotor was put in a bowl of water to cool the content of the tube and also to reduce the associated pressure.The digested soil samples were then filtered using Whatman No 1 filter paper, into 50 mL volumetric flasks and made up to the mark using de-ionized distilled water. The chemicals used were analytical grade chemicals obtained from Sigma Aldrich. The calibration standards for Cd, Pb, As, Cr, Mn, Fe, Cu, and Zn were prepared, and together with the reagent blanks, subjected to same digestion procedure as the samples. Subsequently, the digested standards, reagent blanks, and samples were determined at the respective wavelengths using AAS, model AA240FS. Acetylene gas was used as the carrier gas, while inert argon was pass through the system to remove interfering gases between each reaction time. Samples for Hg were digested by adding 5.0 mL of H2SO4 followed by 2.5 mL of HNO3 and 15.0 mL of freshly prepared 5% (w/v) KMnO4. The mixtures were made to stand for at least 15.0 minutes after which 8.0 mL of 5% (w/v) K2S2O8 solution was added and digested for 25.0 minutes using the operation parameters outlined above for Pb, As and Cd. The samples were decolourised by adding 10% hydroxylamine hydrochloride solution. A blank and calibration standards were prepared. Cold vapour was used for Hg determination using 3% HCl in 1.1% SnCl2 and 3% HCl as the reductant at a wavelength of 253.7 nm [12].Blank samples were also prepared for the other elements for analysis. The reproducibility of the analytical method was validated by analysing standard reference material Soil-7. The precision was calculated as a percentage relative standard deviation (%RSD) of five replicate samples of the prepared standard and was found to be less than 5%.
3. Results and Discussion
3.1. Statistics
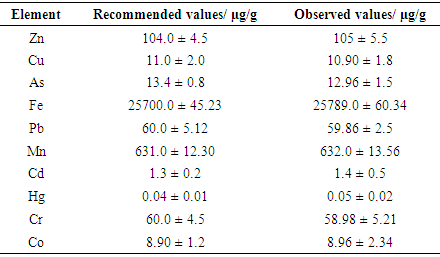
- The reproducibility of the analytical method was assessed by analysis of the standard reference material IAEA soil-7. Table 1 shows the recommended values for Cr, Mn, Zn, Pb, As, Cd, Hg, Cu, Fe and Co in soil-7 against the experimental values obtained using AAS. The values obtained, compared favourably well with the recommended values as Spearman correlation coefficient was +0.96%.
|
|
|
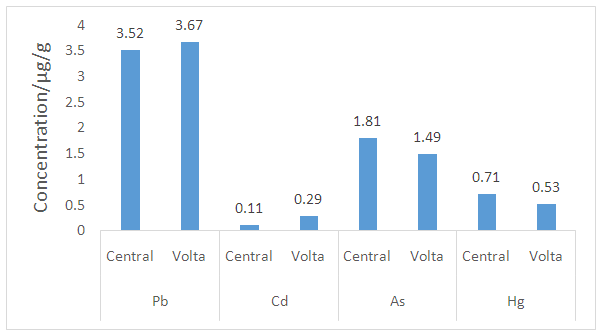 | Figure 1. Relative toxic heavy metal levels in earthenware clay deposits in the Central and the Volta regions of Ghana |
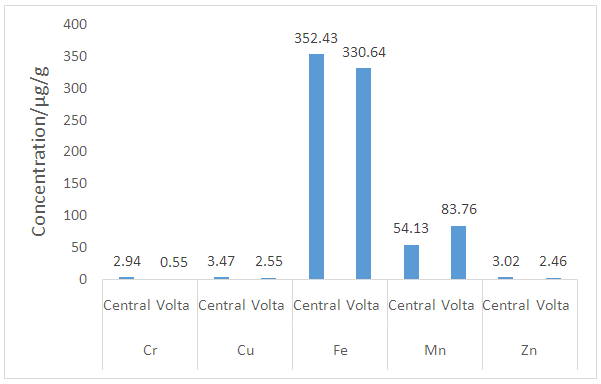 | Figure 2. Relative micronutrient/essential elements levels in earthenware clay deposits in the Central and the Volta regions of Ghana |
4. Conclusions
- Heavy metal levels investigated in earthenware clay deposits from the study regions were similar in Zn, Mn, Cu, Fe, Cd, Pb, and As levels. In most cases, the levels were within the acceptable limits. The levels of these heavy metals were also lower than the levels reported in geophagy clay which is eaten directly.There is, however, statistical differences in Cr and Hg levels in the two regions. The levels of Cr and Hg in the Central region were generally higher than the levels observed in the Volta region. The differences in Cr and Hg levels in the two region might be due to the rampant and indiscriminate use of insecticides and herbicides which is a common practice in the Central region compared to the Volta region. Runoff from agricultural lands might collect these residues from farmlands to the clay deposits over time. In general, the heavy metal content of earthenware clay deposits in the two regions are low and as such the clay deposits can be said to be good raw materials for the production of food -ware products.
ACKNOWLEDGEMENTS
- The author expressed his appreciation to Mr. Bobobee, a colleague at the Chemistry Education Department, University of Education, Winneba for reading the manuscript and making important suggestions.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML