-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2017; 7(4): 105-112
doi:10.5923/j.chemistry.20170704.01

Chemical and Physical Characterization of Moroccan Bentonite Taken from Nador (North of Morocco)
M. El Miz, H. Akichoh, D. Berraaouan, S. Salhi, A. Tahani
Lacprene, Faculté des Sciences Oujda, Université Mohamed 1er, Route de Sidi Maâfa, Oujda, Morocco
Correspondence to: M. El Miz, Lacprene, Faculté des Sciences Oujda, Université Mohamed 1er, Route de Sidi Maâfa, Oujda, Morocco.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Clay samples of bentonite taken at Nador region of North-Eastern Morocco have undergone a series of mineralogical, chemical and physico-chemical analyzes including mineralogical composition (X-ray diffractions and infrared spectroscopy), chemical analysis, cation exchange capacity, specific surfaces and thermal analysis. The crude bentonite contained calcium-rich smectite (>80%). It also contained other mineralogical content of less than 20 by weight, consisting mainly of quartz. The fine fraction (≤ 2 µm) was purified and exchanged with sodium cations. The chemical composition of the fine fractions and the low crystalline phases bound to Fe-, Al- and Si-phases isolated by selective extractions were used in the calculation of chemical formula of smectite clay. The mineralogical and chemical compositions of the samples are typical of bentonites, mainly consisting of montmorillonite. The results revealed that this clay have quality necessary in various applications such as cosmetics, medicinal, paints, nanomaterials etc.
Keywords: Moroccan Bentonite, Clay, Mineralogical composition, Chemical composition, Cation exchange capacity, Specific surface area
Cite this paper: M. El Miz, H. Akichoh, D. Berraaouan, S. Salhi, A. Tahani, Chemical and Physical Characterization of Moroccan Bentonite Taken from Nador (North of Morocco), American Journal of Chemistry, Vol. 7 No. 4, 2017, pp. 105-112. doi: 10.5923/j.chemistry.20170704.01.
Article Outline
1. Introduction
- Geological characteristics of the world bentonite mineral resources are mainly concentrated in magmatic activity (volcanic activity) related areas, the volcano-sedimentary deposit types, the weathered residual and hydrothermal are related to volcanic activity. According to the material, the global bentonite of North African origin is mainly distributed in North East Morocco in the region of Nador city (Figure 1). Industrial Minerals Company is engaged in the production of white bentonite in Nador region, with an annual output of 150,000 tons which represents an estimated value of 1.5% of world coumption. The Nador bentonite deposits are exploited and not industrially used in Morocco but in Europe. The main destination countries for the Nador bentonite were Spain and Greece. These latter’s increase their potential reserve of bentonite for commercial use and export, due to the Moroccan bentonite importation.Commercial importance of bentonites depends on the contents of their clay and nonclay minerals. Dominant clay minerals in bentonites are smectites irrespective of their origin such as montmorillonite, beidellite, saponite and hectorite [1]. Bentonite and their major clay mineral smectites have been among the most important industrial raw material. Some of the applications are civil engineering and environmental ones, animal litter, paint, paper, plastics, decolorization, foundry bondants, drilling fluids, desiccants, sealants, cosmetics, adhesives, and catalysts [2]. The purification and physicochemical modifications of pure smectites have a great importance to prepare some high technology materials such as pillared clays, organo-clays, and polymer/smectite nanocomposites. For many years, the clay materials have been used for adsorption of heavy metals [3, 4], dye molecules [5], herbicides [6], anions such as nitrates [7], like phosphates and sulphates, or gas adsorption [8], like SO2. In industry, these materials are also used as a catalysator in organic syntheses or as excipient in pharmacy. The application of clay materials is greatly governed by their surface properties like adsorption capacities, surface charges, large surface area, charge density, the type of exchangeable cations, hydroxyl groups on the edges, silanol groups of the crystalline defects or broken surfaces and Lewis and Brönsted acidity [9]. Phyllosilicate surfaces contain two basic types, i.e., siloxane surface and hydroxyl surface.The quality of a bentonite that refers to the performance of the material in its different applications depends largely on the quality and quantity of the smectite, which the most common mineral is montmorillonite. Therefore, the isolation of some smectite group minerals from bentonites is of great importance. A specific purification method for each bentonite needs to be developed depending upon the properties of its clay and nonclay minerals. The particle sizes of the smectite particles are smaller than 2 micrometer in aqueous suspensions. This property is of great importance in the purification of bentonites since it permits the separation of smectites from the bentonites. The grade of a bentonite which refers to the smectite content of the bentonite, can be assessed by measurement of the cation exchange capacity (CEC) and/or its total specific surface area usually determined with ethylene glycol.The present paper is the outcome of such an effort conducted to develop the potential use of mineral resources of North East Moroccan clay deposits. The studied Bentonite clay was taken from the deposits of Azzouzete in the district of Nador (Morocco) at 20 Km south of Nador city (35’01’ 56,49° N and 2’52’ 11,37°O with 106 m of Altitude ) Figure 1 et 2.
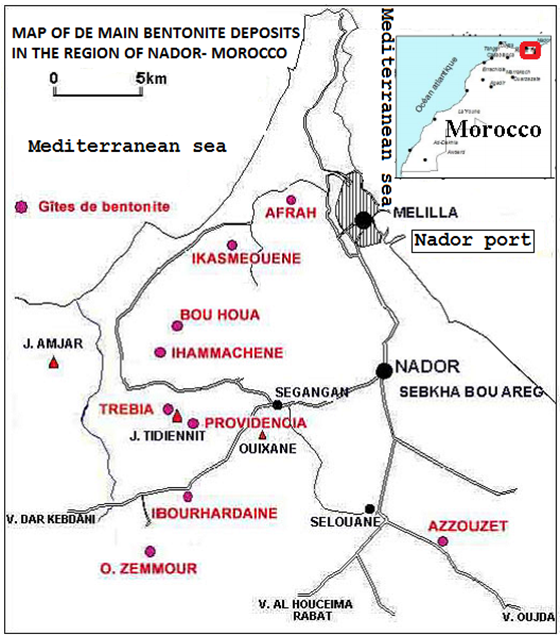 | Figure 1. Location of Nador city: site of the clay samples |
 | Figure 2. Location of the clay sample sites: Nador deposit of Azzouzete (Source: Google Earth 35’01’ 56,49° N and 2’52’ 11,37° O with 106 m of Altitude) |
2. Technics and Methods
- The studied Azzouzete’s bentonite comes from deposit located in the north of Morocco (Nador area). For our study, we collected a mass of clay from 50 Kg to a depth of 6 meters. The bentonite clays of this deposit are essentially white color. The investigated characteristics of chemical analysis and charge surface determination were compared to those of a commercial Wyoming bentonite supplied by the Clay Minerals Society a source clay Wyoming SPV.
2.1. Preparation of Sodium Homo Ionic Bentonite
- We carry out the elimination of all the crystalline phases (quartz, feldspar, calcite…) by a pretreatment of the sample rough by sodic homo-ionization. A series of washing has made it possible to eliminate the impurities and to obtain a well defined granular fraction of a size ≤ 2 µm. In practice, this process consists of dispersing a mass of 1 Kg in 5 liters of distilled water in a solid / liquid report of 20% under agitation for an hour until complete homogenization of the suspension. The obtained mixture was then treated by HCl (0,5 M) to eliminate the carbonate. After washing, hydrogen peroxide (10%) was added to oxidize an eventual organic present matter.Subsequently, we conduct the cation exchange by adding NaCl solution of 1M to have a sodic bentonite (sodic smectite). Agitation-centrifugation wash cycle is repeated 5 times. The dark grey background in the centrifuge tubes is eliminated: it contains the fraction enriched in impurity (quartz, cristalobalite…). The remaining chloride is removed by dialysis.
2.2. Characterization Methods
2.2.1. XRD, IR and Thermal Analysis
- Raw clay and purified samples are subjected to mineralogical, physical analysis and identified by X-Ray diffraction (XRD), infrared spectroscopy (IR) and thermal analysis (ATD and ATG). The XRD analysis was conducted using automatic diffractometer Siemens D500, working on copper Kα1 (1.54 Å) monochromatic radiation. The angular scan was performed in discrete steps of 0.05° (2θ) with a counting time of 5 seconds per step. The material is dried and finely ground and the powder sieved through 0.63 mm sieve was placed directly on a sample holder (plastic). Thermal analysis was conducted by a thermal Analyzer type (STRATON) with a speed of heating of 5°C/min to 1000°C, under a controlled atmosphere of helium. IR spectra have been made using a spectrometer Shimadzu Fourier Transform on a range of 400 to 4000 cm-1 with a resolution of 2 cm-1 and samples were conditioned in dispersion in a KBr Pellet (1/200 in weight).
2.2.2. Nitrogen Adsorption/Desorption
- Adsorption–desorption experiments using N2 were carried out at 77 °K on a Sorptomatic 1900 Carlo Erba porosimeter. Before each measurement, the samples were outgassed at 140°C, and vacuumed for more than 12 hrs. The N2 isotherms were used to determine the specific surface areas (SA) using the BET equation. The t-plot method was used to calculate the micropore volume and micropore area.
2.2.3. Ethylene Glycol and Methylene Blue Adsorption
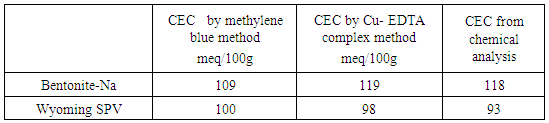
- The cation exchange capacities (C.E.C) were determined by the Copper Ethylene Diamine method Cu-EDA [11] and the total surface was determined by methylene blue adsorption [12]. The total surface area was determined by ethylene glycol gaz adsorption [13].
2.3. Chemical Analysis
- The elementary analysis was conducted by Varian 220 ICP/AES spectrometer. The choice of analytical rays has been done in such a manner to avoid spectral interfaces in the emission of inductive plasma. To perform chemical analysis. The studied clay samples studied were attacked by mixture of (2/3 HCl + 1/3 HNO3). All elements pass in solution except silica evaporates by use of HF.
3. Results and Discussion
3.1. Chemical and Mineralogical Characterization
3.1.1. XRD on Powder
- The sample of initial raw bentonite and Sodium exchanged bentonite (Na-bentonite) were examined by XRD analysis on powder-oriented samples and results are presented in Figure 3.
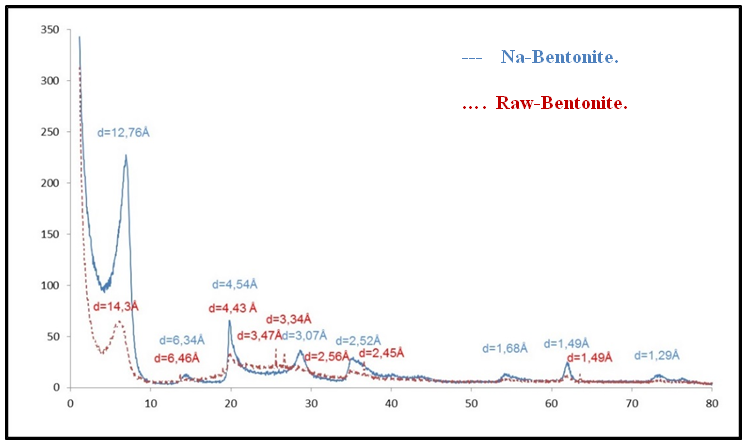 | Figure 3. Diffractograms of the fine fraction powder of Brute and purified Na-Bentonite |
3.1.2. XRD-oriented Slide
- The first blade contains therefore purified bentonite oriented, the second one, the bentonite purified with undergoes heat treatment and the last contains the purified exchanged sodium bentonite.
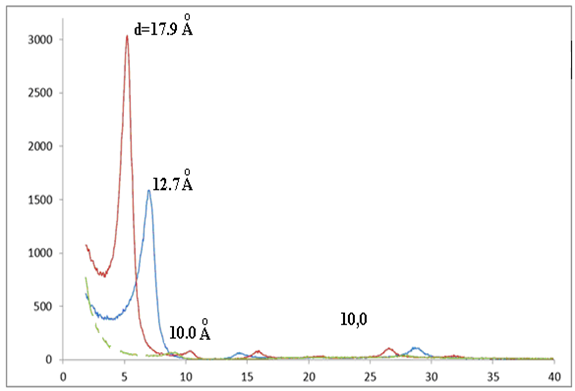 | Figure 4. Diffractograms of oriented aggregates: nontreated; solvated by ethylene glycol; heat-treated at 550 °C for 3 h |
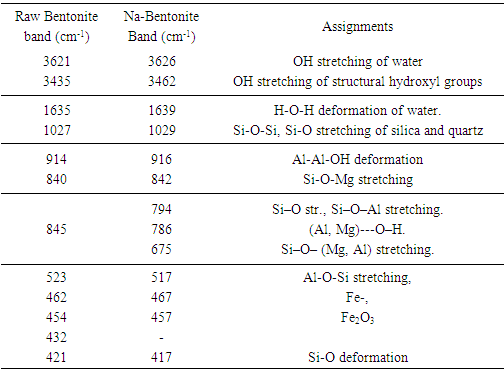
3.1.3. FT-IR Spectroscopy Measurements
- The infrared spectroscopy of the clay samples of raw bentonite and purified sodium bentonite are presented in figure 5 and are almost identical.
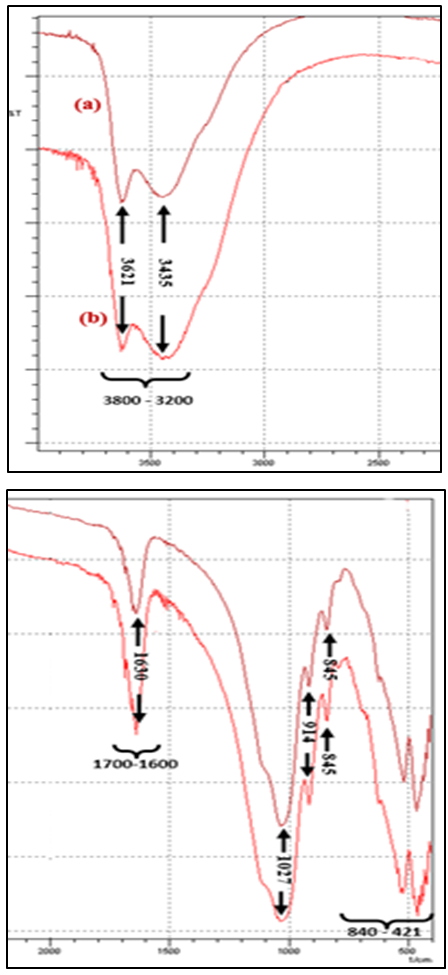 | Figure 5. Infrared spectra: (a) Raw- Bentonite; (b) Na- Bentonite |
|
3.1.4. Thermal Analysis: ATD and ATG
- In general, the analyzed bentonites of Raw and exchanged sodium bentonite showed similar behavior characteristic of 2/1 dioctaedral clays as shown in figure 6. This showed that the studied bentonite has similar structure to montmorillonite [16].
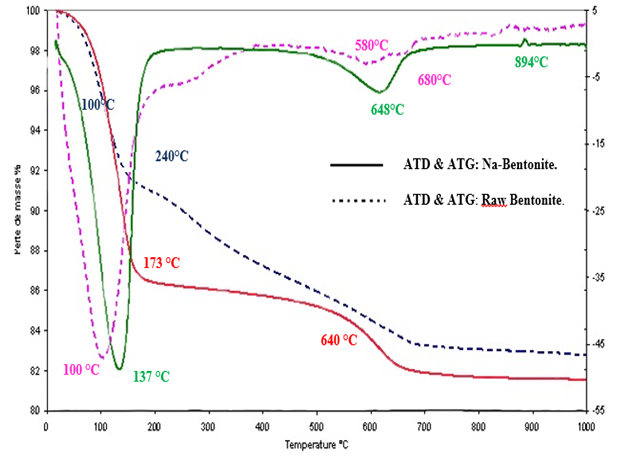 | Figure 6. Thermal analysis: (a) Raw Bentonite; (b) Na-Bentonite |
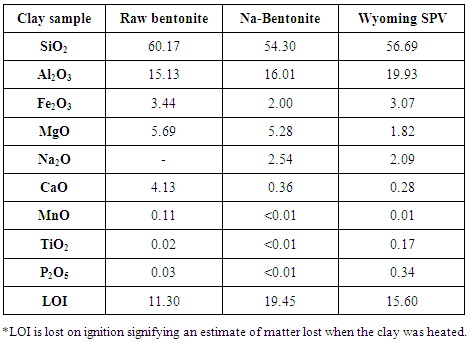
3.1.5. Chemical Analyses
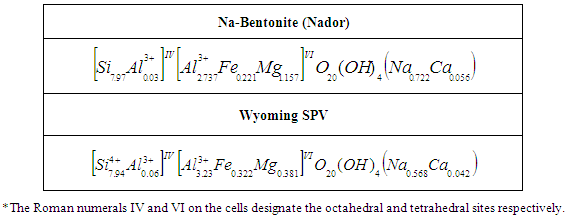
- Elemental analysis of the studied clay samples studied showed that they are composed essentially of Si, Al, Fe, Mg, O, Na and Ca. The elements present in clays have been presented as relative percentages of the elements expressed as oxides and expressed as relative oxide percent by weight in the entire sample in Table 2.
|
|
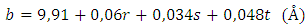 | (1) |
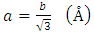 | (2) |
|
|
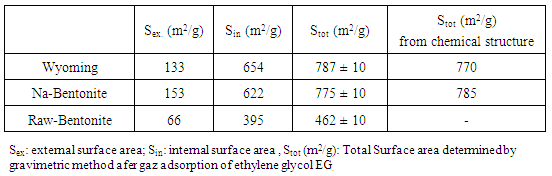
3.1.6. Determination of Bentonite Surfaces
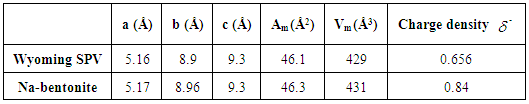
- The measurement of specific surface brings to clays of both mineralogical and textural information. In general, it uses two approaches to determine the specific surface of clays: 1- The adsorption of single molecules, such as nitrogen low temperature to determine the outer surface using data of adsorption isotherm and applying the theory of BET [20].2- The measurement of the total surface area by adsorption of polar liquid (Liquor), its advantage is the capacity to swell the mineral impurities and replace them, which is not the case for nitrogen adsorption method [21]. In this case, Eltantawy Arnold [13] proposed a protocol that measures surfaces for which the molecular size is independent of the nature of the cation and equal to 2.22 m2/mg ethylene glycol (EG). a. Nitrogen adsorption and desorption isothermsThe adsorption-desorption isotherm of nitrogen on Raw and Nador Na-bentonite are shown in Figure 7.
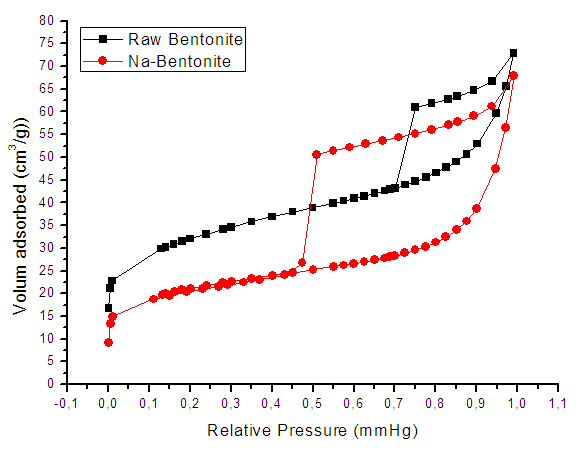 | Figure 7. Adsorption-desorption isotherms of nitrogen on Raw and Na-bentonite |
|
4. Conclusions
- X-ray diffraction, infrared spectroscopy and thermal analysis show that the gross Nador bentonite is a di-octahedral bentonite (montmorillonite type) in calcium form that contains very few impurities as quartz and calcite. The physicochemical characterization of purified and sodium exchanged Nador bentonite compared to the reference Wyoming bentonite SPV shows that it is a di-octahedral clay of montmorillonite type and also presents a very similar chemical composition and physical-chemical characteristics by reference Wyoming montmorillonite SPV. By means of Chemical Analysis, it can be said that the smectites that constitute Nador bentonites are montmorillonites rich in magnesium at the octahedral site. The high montmorillonite content (>80%) of Nador bentonite and its chemical and physical properties determine its great commercial value and potential use in a wide variety of technical applications.
ACKNOWLEDGMENTS
- This work was supported by CNRST under grant no: Protars P23/66 and MESRST under grant no PPR/2015/17.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML