-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2017; 7(3): 97-104
doi:10.5923/j.chemistry.20170703.03

Triterpenes from Elaeodendron schweinfurthianum and Their Antimicrobial Activities against Crop Pathogens
Sylvia Awino Opiyo 1, Lawrence Onyango Arot Manguro 2, Philip Okinda Owuor 2, Elijah M. Ateka 3
1Department of Physical Sciences, Murang’a University, Murang’a, Kenya
2Department of Chemistry, Maseno University, Maseno, Kenya
3Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya
Correspondence to: Sylvia Awino Opiyo , Department of Physical Sciences, Murang’a University, Murang’a, Kenya.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Phytochemical evaluation of Elaeodendron schweinfurthianum (Loes) extracts led to the isolation of nine compounds which were identified as 3-oxofriedelane (1), 3α-hydroxyfriedelane (2), 3-oxo-29-hydroxyfriedelane (3), 3-oxofriedelan-28-al (4), α-amyrin (5), α-amyrin acetate (6), β-sitosterol (7), stigmasterol (8) and lanosterol (9). The structures of the compounds were determined using spectroscopic and physical methods as well as by comparison with literature data. The in vitro antimicrobial activities of the extracts and isolates were investigated against fungi and bacteria which infect food crops. All the crude extract inhibited the growth of the tested pathogens with EtOAc and n-hexane extracts being the most active with 11.3 and 7.3 mm diameter zone of inhibition respectively. All the compound showed antimicrobial activity except compounds 3 which did not exhibit any visible activity at concentrations ≤ 200 µg/ml. Finding from this study confirm that plant extracts can provide alternative readily available and environmentally safe antimicrobials for managing crop infections.
Keywords: Elaeodendron schweinfurthianum, Celastraceae, Triterpenes, Antibacterial, Antifungal
Cite this paper: Sylvia Awino Opiyo , Lawrence Onyango Arot Manguro , Philip Okinda Owuor , Elijah M. Ateka , Triterpenes from Elaeodendron schweinfurthianum and Their Antimicrobial Activities against Crop Pathogens, American Journal of Chemistry, Vol. 7 No. 3, 2017, pp. 97-104. doi: 10.5923/j.chemistry.20170703.03.
Article Outline
1. Introduction
- Globally, food scarcity is the third most pressing problem after poverty [1]. Approximately one billion people are faced by severe hunger worldwide of which 10% actually die from hunger-related complications [1]. This problem arises due to inadequate management methods of pest and microbes-induced spoilages of agricultural produce [2]. Bacterial and fungal infection of agricultural crop can cause up to 100% loses [3-5]. Microbes belonging to several genus including Alternaria, Aspergillus, Fusarium, Rhizopus, Ralstonia and Streptomyces cause infection on crops both in storage and in the field [2]. These pathogens cause disease in a wide range of crops including cereals, tubers, fruits, and vegetables as well as ornamental crops [3-6].Synthetic chemicals such as dichloronitroaniline have been used to protect crops against microbial infections [7]. However, the use of such chemicals apart from their potential danger to both for humans and environment [8, 9] are unaffordable by most farmers. Moreover, because of pathogens resistance, most chemicals have become ineffective [9]. Most crop infecting microbes have a wide host range which further complicates their control. In order to eliminate agricultural produce spoilage, there is a need to search for affordable, readily available, sustainable, and environmentally friendly means of managing the problems posed by these pathogens. Plants belonging to the genus Elaeodendron (Celastraceae) are characterized by the presence of terpenoids, steroids and flavonoids [10-15]. Biological activities of Elaeodendron species include antifungal, antibacterial, feeding deterrent, cytotoxic and antiviral [15-18]. E. schweinfurthianum (Loes) which is widely distributed in tropical Africa is used traditionally to manage bacterial and fungal infections including wounds, primary symptoms of syphilis and diarrhea [19, 20]. In this communication, we report the isolation of 3-oxofriedelane (1), 3α-hydroxyfriedelane (2), 3-oxo-29-hydroxyfriedelane (3), 3-oxofriedelan-28-al (4), α-amyrin (5), α-amyrin acetate (6), β-sitosterol (7), stigmasterol (8) and lanosterol (9) from E. schweinfurthianum. These compounds together with their activities are being reported from this plant for the first time.
2. Materials and Methods
2.1. General
- Melting points were determined on a Gallenkamp (Loughborough, UK) melting point apparatus and are uncorrected. The UV spectra were run on Pye Unicam SP8-150 UV–vis spectrophotometer (Cambridge, UK) using acetonitrile. IR data were recorded on a PerkinElmer FTIR 600 series spectrophotometer (Waltham, MA, USA) as KBr pellet. The 1H and 13C NMR data were measured in CDCl3 and CDCl3–DMSO-d6 on a Bruker NMR Ultrashield TM (Darmstadt, Germany) operating at 500 and 125 MHz, respectively. The MS data were obtained on a Varian MAT 8200A instrument (Bremen, Germany).
2.2. Plant Materials
- Stem bark of E. schweinfurthianum was collected from Shimba Hills (latitude 4° 15' 53.84'' S and longitude 39° 22' 19.61'' E) in September 2008 and voucher specimen (2008/09/04/SAO/CHEMMK) was identified at the Kenya National Museum herbarium after comparison with authentic samples. The plant materials were chopped into small pieces, air dried and ground into fine powder using a mill.
2.3. Extraction and Isolation of Compounds
- Powdered plant material (2 kg) was extracted sequentially with n-hexane, EtOAc and MeOH by soaking the material in the solvent for seven days. The mixture was filtered and solvent evaporated at reduced pressure to yield 15 g, 100 g and 210 g of n-hexane, EtOAc and MeOH extracts, respectively. n-Hexane extract (10 g) was chromatographed over silica gel-packed column (2.5 x 60 cm, 150 g) and eluted with n-hexane - ethyl acetate mixture to yield 100 fractions each of 20 ml. Fractions showing similar TLC profiles were combined resulting into three pools (I-III). Pool I (1 g) did not show any major spot on TLC and was discarded. Pool II (3 g) crystallized out to give a white compound which on further purification using n-hexane-ethyl acetate (9:1) gave α-amyrin acetate (6) 56 mg. The mother liquor of this pool was subjected to further column chromatography with n-hexane- EtOAc (9:1) to afford stigmasterol (8) 78 mg. Pool III (3 g) also crystallized out and after re-crystallization (n-hexane-EtOAc, 9:1) afforded further stigmasterol (8) 45 mg. Ethyl acetate extracts (75 g) was chromatographed over silica gel-packed column (5 x 60 cm, 200 g) eluting with n-hexane-ethyl acetate (10% increment of ethyl acetate), ethyl acetate neat and finally with CH2Cl2-MeOH (with 10% and 20% increment of MeOH) to yield 251 fractions (20 ml each). Fractions showing similar TLC profiles were combined resulting in five pools (I-V). Pool I (8 g) on subjection to further column chromatography eluting with n-hexane-ethyl acetate (95:5, 9:1) gave α-amyrin acetate (6) 30 mg. Pool II (15 g) on further fractionation with n-hexane: ethyl acetate mixture (95:5, 9:1, 4:1) afforded α-amyrin acetate (6) 72 mg, 3-oxofriedelane (1) 65 mg and β-sitosterol (7) 54 mg. Pool III (17g) yielded stigmasterol (8) 78 mg, 3-oxofriedelan-28-al (4) 80 mg and 3α-hydroxyfriedelane (2) 83 mg. Pool IV (13 g) afforded α-amyrin (5) 77 mg and 3-oxo-29-hydroxyfriedelane (3) 93 mg on further fractionation with n-hexane: ethyl acetate mixture (4:1, 7:3). Pool V (9 g) gave lanosterol (9) 74 mg on further column chromatography eluting with n-hexane: ethyl acetate (7:3, 3:2).
2.4. Antimicrobial Assay
- Test organisms were isolated from infected farm produce obtained from local market were tested for antimicrobial activity as described by Barry et al., [21]. Inoculation was done by spreading the test pathogen on the surface of the solidified agar. Sterile paper discs (Whatmann No. 1, 5 mm diameter) containing 100 µL of the plant extracts (5 mg/ml) were placed on PDA and NA plates previously inoculated with test fungi and bacteria, respectively and incubated at 28°C for 48 h for fungi and 37°C for 24 h for bacteria. Blitox and streptocycline (10 mg/ml) were used as positive controls while DMSO without plant extract was used as a negative control. The minimum inhibitory concentrations (MICs) of pure isolates were determined according to Kariba et al., [22]. Isolated compounds were tested at concentrations ranging between 1-200 µg/ml. MIC was regarded as the lowest concentration that produced a visible zone of inhibition.
3. Results and Discussion
3.1. Isolated Compounds
- Phytochemical evaluation of the plant yielded nine compounds (Fig. 1) whose structures were determined using spectroscopic methods. EIMS spectrum of compound 1 gave a molecular ion peak at m/z 426 corresponding to C30H50O and was supported by the 13C NMR and DEPT spectra which showed the presence of 30 carbons attributed to eight methyl, eleven methylene, four methine and seven quaternary carbon atoms. The 13C NMR spectra showed the presence of a carbonyl carbon at δ 213.24 and eight methyl carbon atoms peaks at δ 6.81, 14.59, 17.91, 18.63, 20.21, 32.05, 31.73 and 34.94 [23-25]. 1H NMR spectrum showed the presence seven singlets (δ 0.70, 0.84, 0.90, 0.90, 0.98, 1.04 and 1.16) and one doublet (δ 0.86, J =7.0 Hz), integrating for three protons each confirmed the presence of the eight methyl groups [24, 26, 27]. EIMS spectrum of 1 further revealed fragmentation pattern typical of a saturated triterpene as evidenced by characteristic daughter ions at m/z 411 [M-15]+, 344, 273, 205 and 123 [24]. Based on the spectral data as well as comparison with literature data, compound 1 was identified as 3-oxofriedelane.
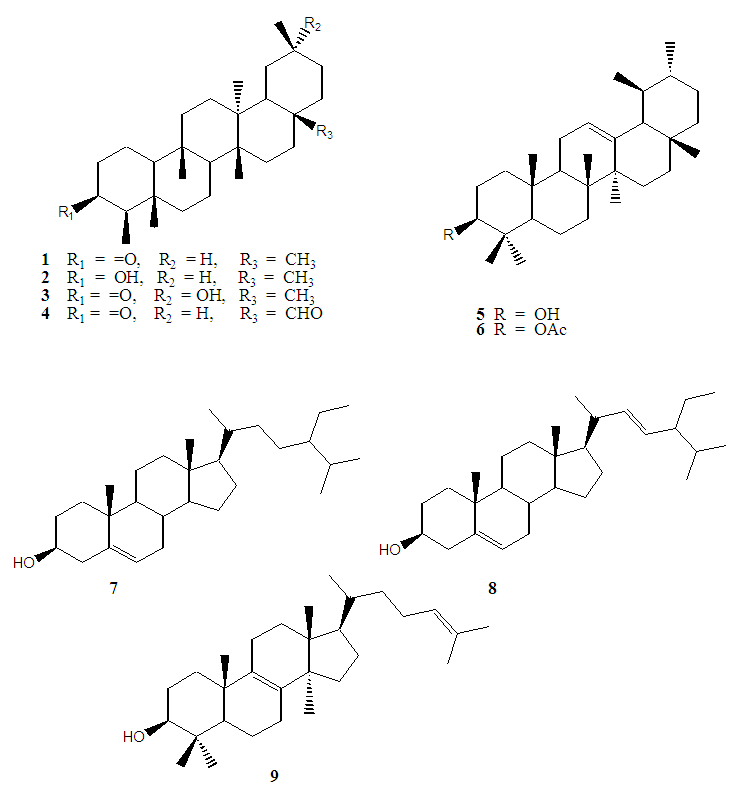 | Figure 1. Structures of compounds isolated from E. schweinfurthianum |
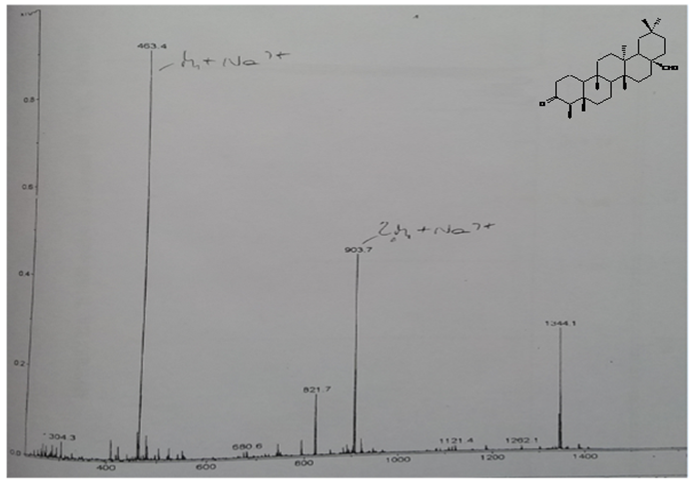 | Figure 2. Mass spectrum of compound 4 |
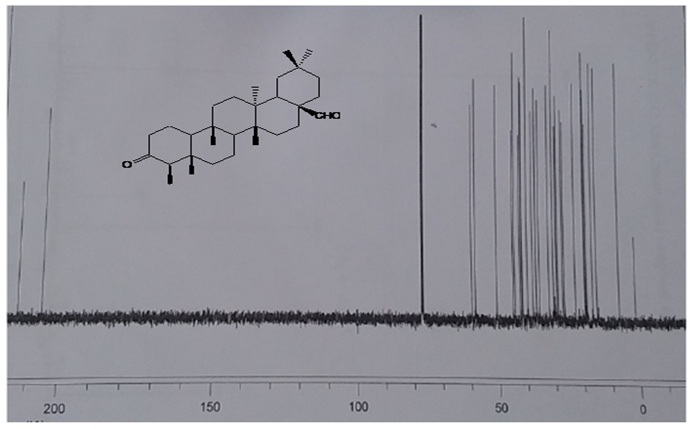 | Figure 3. 13C NMR spectrum of compound 4 (CDCl3, 90MHz) |
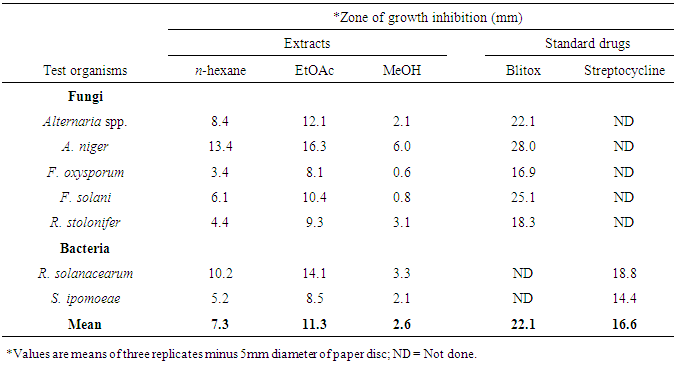
3.2. Antimicrobial Activities of Extracts and Isolates
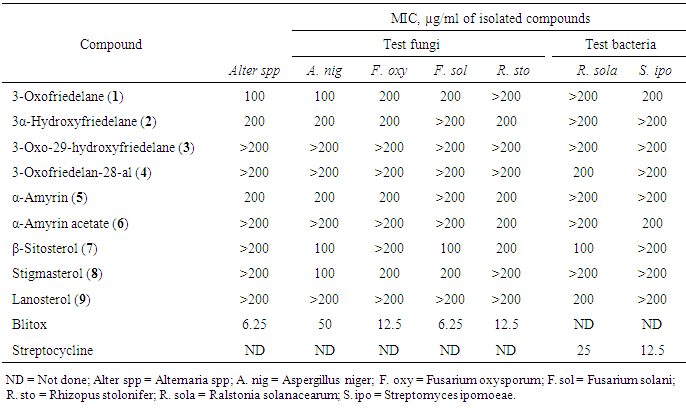
- Crude extracts and isolates from stem bark of E. schweinfurthianum were subjected to antimicrobial assays against sweet potato pathogens: Alternaria spp, Aspergillus niger, Fusarium oxysporum, F. solani, Rhizopus stolonifer, Ralstonia solanacearum and Streptomyces ipomoeae. All the extracts were active against the fungi and bacteria species tested (Table 1). Ethyl acetate extract was the most active (p ≤ 0.05) against the pathogens followed by n-hexane extract. The most susceptible fungi to EtOAc extract was A. niger (inhibition zone, 16.3 mm) while F. oxysporum was least susceptible to the extract (inhibition zone, 8.1 mm). In the antibacterial test, R. solanacearum was more susceptible EtOAc extract (inhibition zone, 14.1 mm) than S. ipomoeae.All the compound were active against one or more of the seven pathogens tested except compounds 3 which did not exhibit any visible inhibition at concentrations ≤ 200 µg/ml (Table 2). 3-Oxofriedelane (1) inhibited the growth of all the pathogens except R. stolonifer and R. solanacearum while α-amyrin (5) inhibited the growth all except F. solani, and R. solanacearum. 3-oxofriedelan-28-al (4) and lanosterol (9) were only active against R. solanacearum. 3-oxofriedelane (1) was the most active against Altenaria spp (MIC = 100 µg/ml) 3-oxofriedelane (1), β-sitosterol (7) and stigmasterol (8) were the most active against A. niger (MIC = 100 µg/ml). F. solani, and R. solanacearum were most susceptible to β-sitosterol (7) MIC = 100 µg/ml) compared to the other isolates. The results from this study confirm that plant infections can be managed using herbal extracts as had also been observed in other studies [43-45]. The herbal extracts are more environmentally safe compared to the synthetic antimicrobial drugs currently used. Further studies to determine the synergism effect of the isolated compound against the test microorganisms is recommended.
|
|
ACKNOWLEDGEMENTS
- The authors are thankful to Kenya Medical Research Institute (KEMRI), Kisumu, Kenya for the use of their laboratory to perform the biological activity tests and Biosciences Eastern and Central Africa Network (BecANet) for financial support. Mr. Mutiso of Botany Department, Nairobi University is highly thanked for identification of the plant.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML