-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2017; 7(3): 67-72
doi:10.5923/j.chemistry.20170703.01

Pyrolytic Extraction and Evaluation of Tar from Chikila Coal and Its Application in Shampoo Production
Ibrahim Iliya Nkafamiya1, Sori Ronald Makan2, Ayodele Akinterinwa1, Ago Mikyitsabu Atoshi1
1Modibbo Adama University of Technology, Yola, Nigeria
2National Metallurgical Development Center, Jos, Nigeria
Correspondence to: Ayodele Akinterinwa, Modibbo Adama University of Technology, Yola, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

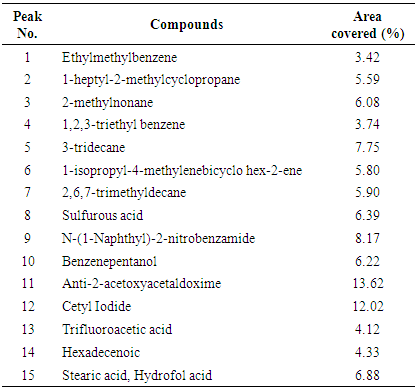
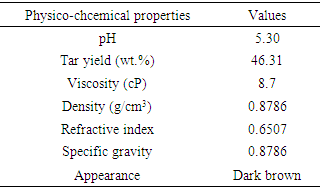
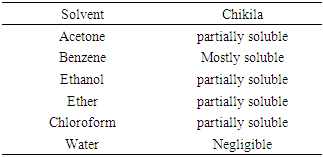
Extraction of tar by pyrolysis from Chikila (CHI) coal and its application in shampoo production was the major aim of this reseacrch. To evaluate the chemical composition of the tar, it was subjected to GC-MS analysis and this showed that CHI tar contains 42 chemical compounds. Anti-2-acetoxyacetaldoxime covered an area of 13.62%. Ethylmethylbenzene which is an important chemical compound, covers an area of 3.42%. Physical property tests present the physical appearance of the coal tar extracted to be dark brown. The pH value was 5.3, density was 0.8786 g/mL, refractive index was 0.6507 and specific gravity was 0.8786. Solubility test showed that CHI tar, is mostly soluble in benzene, partially soluble in ethanol, ether and chloroform, but its solubility in water was negligible. The tar was applied in a tailored production of shampoo. Shampoo produced was subjected to quality assessment to establish the potentials of the tar as a raw material in a cosmetic industry.
Keywords: Coal, Tar, Pyrolysis, Chikila, Cosmetics
Cite this paper: Ibrahim Iliya Nkafamiya, Sori Ronald Makan, Ayodele Akinterinwa, Ago Mikyitsabu Atoshi, Pyrolytic Extraction and Evaluation of Tar from Chikila Coal and Its Application in Shampoo Production, American Journal of Chemistry, Vol. 7 No. 3, 2017, pp. 67-72. doi: 10.5923/j.chemistry.20170703.01.
1. Introduction
- Coal pyrolysis is one of the significant approaches for the comprehensive utilization of coal, and one important product of the process is coal tar. Coal tar can be utilized as raw materials for various industries such as synthetic fiber, medicines, coatings and national defense (Jiang et al., 2006). Coal is one of the most abundant fossil fuels in Nigeria. Globally, it is one of the leading commodities in terms of industrial importance and monetary value (Jauro et al., 2008; Ryemshak and Jauro, 2013). It is a type of raw materials from which phenols, naphthalenes, anthracene can be extracted for the production of washing oil, cementitious agents, antiseptic agents and catalyst for catalytic hydrogenation to produce gasoline, diesel oil etc (Jauro et al., 2006).The pyrolytic behavior of coal is known to be affected by the temperature, heating rate, particle size, pressure, coal type and many other factors (Smith et al., 1994). As the coal particle temperature rises during the pyrolysis process, the bonds between the aromatic clusters in the coal macromolecule break, creating fragments that are detached from the macromolecule. The larger fragments are referred to as metaplas (Smith et al., 1994).The pyrolytic behavior of a coal is a strong function of coal type or rank. Low rank coals, such as lignite and sub-bituminous produce relatively high levels of light gases and very little tar. Bituminous coals produce significantly more tar than the low rank coals and moderate amounts of light gases. The higher rank coals produce relatively low levels of both light gases and tar. Coal tar extraction can be carried out by either solvent extraction method or pyrolysis method. The method of extraction used in this research work is the pyrolysis method. There are also different types of coal pyrolysis processes, and they are as follows: Conventional pyrolysis process, Fluidized pyrolysis process and fixed bed pyrolysis process. The fixed bed pyrolysis process was chosen for this work because it is easy to construct, cheap and requires less maintenance (Musale et al., 2013). There are many discovered coal deposit in Nigeria which are never exploited or investigated, and the deposit in Chikila is one of these (Jauro et al., 2008). Coal tar has been found to be a valuable raw material in the production of a wide range of industrial materials, and these includes; soap, ointment, paint, synthetic fibers, dyes, photographic materials, detergentsand medicated shampoo which is used in the treatment of dandruff and psoriasis, as well as being used to kill and repel head lice (Van-Metre and Mohler, 2010).In this study, the tar extracted from a virgin coal deposit in Chikila will be analyzed to establish its quality. The product will then be used as a raw material in an optimized production process of an industrial product (shampoo). These will present the potentials of the mineral deposit and propose its economic utilization.
2. Materials and Methods
- This research work was conducted in the chemical engineering laboratory, University of Jos, Nigeria. Chemicals used which includes; acetone, benzene, chloroform, citric acid, ethanol, ether, alkyl benzene sulfunate, sodium chloride are all analytical graded product of the British Drug House. Coconut oil, perfume, aloe vera and colorants (green, golden and blue) are bought from the market in Jos, Nigeria. All products are used as obtained without any treatments. Sample collection and preparationChikila coal deposit is along the Benue trough in the Village called Chikila, located in the outskate of Guyuk town, in Guyuk Local Government Area of Adamawa State, North-Eastern Nigeria. Coal sampling was done on the 15th of June, 2016. The sampling methods used were from the work by Ryemshak and Jauro, (2008). Five samples were collected. These were subjected to further preparatory processes which includes; drying (air drying) followed by pulverization in a mortar, then sieving through a 250 micron mesh, to obtain the sample that was finally subjected to the pyrolytic extraction of the coal tar. Coal tar extraction The fixed bed pyrolysis method was chosen for this work (Musale et al., 2013). Fixed bed pyrolysis is a medium temperature process in which the feedstock (coal) was heated in a reactor in the absence of air, partly vaporizes and condenses to form a mixture of dark brown liquid (tar), water, gases and the other part residue (char) (Kumar and Mali, 2009).Analysis of TarDetermination of tar chemical compositionsChemical components of the coal tar were determined using GCMS-QP2010 SHIMADZUJAPAN. GC/MS is a combination of two different analytical techniques, Gas Chromatography (GC) and Mass Spectrometry (MS), was used to analyze complex organic and biochemical mixtures. GC separates volatile and semi volatile compounds with great resolution. MS can provide detailed structural information on most compounds such that they can be exactly identified and quantified.Data for the GC-MS was displayed in a total-ion chromatogram, which sums the total ion abundances in each spectrum and plots them as a function of time. Another is the mass spectrum at a particular time in the chromatogram to identify the particular component that was eluted at that time. Determination of tar yield The method of tar yield determination was a modification of the one adopted by Adeleke and co-workers, (2007). The percentage coke and tar yields were calculated using the formula respectively.
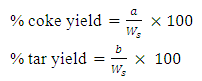 where, a = weight of semi coke residue in the test tubeb = weight of tar and water collected andWs = weight of sampleDetermination of tar viscosityASTM D166 was used for tar viscosity test. The viscosity of the coal tar samples was determined using the Brookfield dial viscometer (Model DV-1, LVT). The viscosity of the sample was measured and recorded directly in cP (Sharma, 1998).Determination of tar solubility (ASTM-E1148)Using the test method described by Nadkarmi, (2007), the solubility of the tar sample was tested by dissolving it in Acetone, Benzene, chloroform, ethanol, ether and Water.Determination of tar pHUsing digital pH Meter (model 250 pH), the pH of the tar sample was measured at room temperature, 25°C Sharma (Peter et al., 2012).Determination of tar refractive indexThe refractive index of the tar sample was measured and recorded with Abbe refractmeter according to standard method (AOAC, 2000).Determination of tar densityThe tar density test in ASTM D70 was used. Empty Hubbard Karmic, 24 mL capacity density bottle was washed, rinsed with water then with ethanol. The bottle was dried in a hot air oven and cooled in a desiccator and weighed. The tar sample, heated at 40°C was introduced into the empty bottle filled to the neck and tightly closed with the stopper allowed to cool down to room temperature and weighed. The test was carried out at a temperature of 25°C. The density was calculated using the equation below.
where, a = weight of semi coke residue in the test tubeb = weight of tar and water collected andWs = weight of sampleDetermination of tar viscosityASTM D166 was used for tar viscosity test. The viscosity of the coal tar samples was determined using the Brookfield dial viscometer (Model DV-1, LVT). The viscosity of the sample was measured and recorded directly in cP (Sharma, 1998).Determination of tar solubility (ASTM-E1148)Using the test method described by Nadkarmi, (2007), the solubility of the tar sample was tested by dissolving it in Acetone, Benzene, chloroform, ethanol, ether and Water.Determination of tar pHUsing digital pH Meter (model 250 pH), the pH of the tar sample was measured at room temperature, 25°C Sharma (Peter et al., 2012).Determination of tar refractive indexThe refractive index of the tar sample was measured and recorded with Abbe refractmeter according to standard method (AOAC, 2000).Determination of tar densityThe tar density test in ASTM D70 was used. Empty Hubbard Karmic, 24 mL capacity density bottle was washed, rinsed with water then with ethanol. The bottle was dried in a hot air oven and cooled in a desiccator and weighed. The tar sample, heated at 40°C was introduced into the empty bottle filled to the neck and tightly closed with the stopper allowed to cool down to room temperature and weighed. The test was carried out at a temperature of 25°C. The density was calculated using the equation below.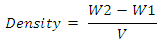 Where W2 = weight of bottle filled tarW1 = weight of empty bottleV = volume of tar in the bottle Production of CHI tar Shampoo Hot water 75 mL (70°C) was measured into mixing vessel (a plastic bowl), 11.5 g of surfactant (alkyl benzene sulphonate) was weighed using a digital electronic balance, transferred into the vessel and mixed. 1 g of tar (active ingredient) was added and stirred, 1.5 g of Aloe Vera was added which was stirred again. 5 mL of hydroxyethylcellulose alongside with 1 mL of perfume, 1 mL of (green) coloring agent was added, and then 2 mL of formaldehyde was also added. Finally, the pH of the mixture was adjusted to 6.5, using citric acid, with greater stirring until a homogenous paste was obtained (Mottram and Less).This whole process was repeated for 0.2 g, 0.5 g, 1.0 g 1.5 g and 2.0 g of the tar (active ingredient).Quality Assessment of CHI tar ShampooTo evaluate the prepared formulations, quality control tests including visual assessment and physicochemical controls such as pH, Solid contents, specific gravity and viscosity, was performed (Aghel et al., 2007; Peter et al., 2012).Determination of CHI tar shampoo pH The pH of 10% shampoo solution in distilled water was determined at room temperature 25°C using a pH meter (Peter et al., 2012).Determination of CHI tar shampoo viscosityThe tar viscosity test in ASTM D166 was used. The viscosity of the shampoo sample was determined using the Brookfield dial viscometer (Model DV-1, LVT USA). The sample was placed in a small sample adapter, and then transferred into the sample cup of the viscometer. The viscometer was set at different spindle speed from 0.3 to 100 rpm. The viscosity of the tar was measured using spindle T95. The temperature was kept constants (45°C), during the study. The viscosity of the sample was measured and recorded directly in cP (Peter et al., 2012). This process was conducted for the different tar concentrations.Determination of specific gravity in CHI tar shampooSpecific gravity was determined using specific gravity bottle and according to the procedure described by Standard Organization of Nigeria (SON, 2009). Empty specific gravity bottle was washed, rinsed with water then with ethanol. The bottle was dried and weighted. The empty bottle was filled with distilled water, closed with a stopper, thoroughly wiped with a clean towel and weighed. After this, the water was decanted and the bottle dried in a hot air oven and was cooled in a desiccator. The shampoo sample was introduced into the bottle filled to the neck and tightly closed with the stopper and weighed. The test was carried out at a temperature of 25°C. The specific gravity was calculated using the formula below
Where W2 = weight of bottle filled tarW1 = weight of empty bottleV = volume of tar in the bottle Production of CHI tar Shampoo Hot water 75 mL (70°C) was measured into mixing vessel (a plastic bowl), 11.5 g of surfactant (alkyl benzene sulphonate) was weighed using a digital electronic balance, transferred into the vessel and mixed. 1 g of tar (active ingredient) was added and stirred, 1.5 g of Aloe Vera was added which was stirred again. 5 mL of hydroxyethylcellulose alongside with 1 mL of perfume, 1 mL of (green) coloring agent was added, and then 2 mL of formaldehyde was also added. Finally, the pH of the mixture was adjusted to 6.5, using citric acid, with greater stirring until a homogenous paste was obtained (Mottram and Less).This whole process was repeated for 0.2 g, 0.5 g, 1.0 g 1.5 g and 2.0 g of the tar (active ingredient).Quality Assessment of CHI tar ShampooTo evaluate the prepared formulations, quality control tests including visual assessment and physicochemical controls such as pH, Solid contents, specific gravity and viscosity, was performed (Aghel et al., 2007; Peter et al., 2012).Determination of CHI tar shampoo pH The pH of 10% shampoo solution in distilled water was determined at room temperature 25°C using a pH meter (Peter et al., 2012).Determination of CHI tar shampoo viscosityThe tar viscosity test in ASTM D166 was used. The viscosity of the shampoo sample was determined using the Brookfield dial viscometer (Model DV-1, LVT USA). The sample was placed in a small sample adapter, and then transferred into the sample cup of the viscometer. The viscometer was set at different spindle speed from 0.3 to 100 rpm. The viscosity of the tar was measured using spindle T95. The temperature was kept constants (45°C), during the study. The viscosity of the sample was measured and recorded directly in cP (Peter et al., 2012). This process was conducted for the different tar concentrations.Determination of specific gravity in CHI tar shampooSpecific gravity was determined using specific gravity bottle and according to the procedure described by Standard Organization of Nigeria (SON, 2009). Empty specific gravity bottle was washed, rinsed with water then with ethanol. The bottle was dried and weighted. The empty bottle was filled with distilled water, closed with a stopper, thoroughly wiped with a clean towel and weighed. After this, the water was decanted and the bottle dried in a hot air oven and was cooled in a desiccator. The shampoo sample was introduced into the bottle filled to the neck and tightly closed with the stopper and weighed. The test was carried out at a temperature of 25°C. The specific gravity was calculated using the formula below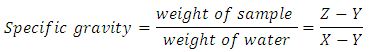 Where x = weight of bottle filled watery = weight of empty bottlez = weight of bottle filled with shampoox – y = weight of water onlyz – y = weight of shampoo only (SON, 2009).This was repeated for the different tar concentrations.Determination of solid contents of CHI tar shampooA clean dry evaporating dish was weighed and 4 g of shampoo was added to it. The dish and shampoo was placed on the hot plate until the liquid portion was evaporated. The weight of the shampoo only (solids) after drying was calculated using the formula below (SON, 2009).
Where x = weight of bottle filled watery = weight of empty bottlez = weight of bottle filled with shampoox – y = weight of water onlyz – y = weight of shampoo only (SON, 2009).This was repeated for the different tar concentrations.Determination of solid contents of CHI tar shampooA clean dry evaporating dish was weighed and 4 g of shampoo was added to it. The dish and shampoo was placed on the hot plate until the liquid portion was evaporated. The weight of the shampoo only (solids) after drying was calculated using the formula below (SON, 2009).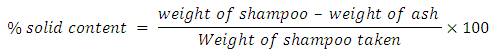 This was repeated for the different tar concentrations.
This was repeated for the different tar concentrations.3. Results and Discussions
- Chromatogram of CHI tarFigure 1 presents the spectra for the GC-MS analysis of Chikila coal tar. The result showed that the tar contains 42 chemical compounds (Table 1 highlight the important chemicals significantly present in the tar). Anti-2-acetoxyacetaldoxime covered an area of 13.62%. The ethylmethylbenzene which has been an important chemical compound for this research covers an area of 3.42%. Chikila coal tar contains other chemicals as showed by the chromatogram, N-(1-Naphthyl)-2-nitrobenzamide, benzenepentanol and 1,2,3-triethylbenzene showed an area covered of 8.17%, 6.22% and 3.74% respectively. Naphthalene compounds have the highest percentage of 37.9%. This confirms Jeffrey’s (2010) report that the naphthalene compound is the most dominant volatile component of PAHs in coal tar.
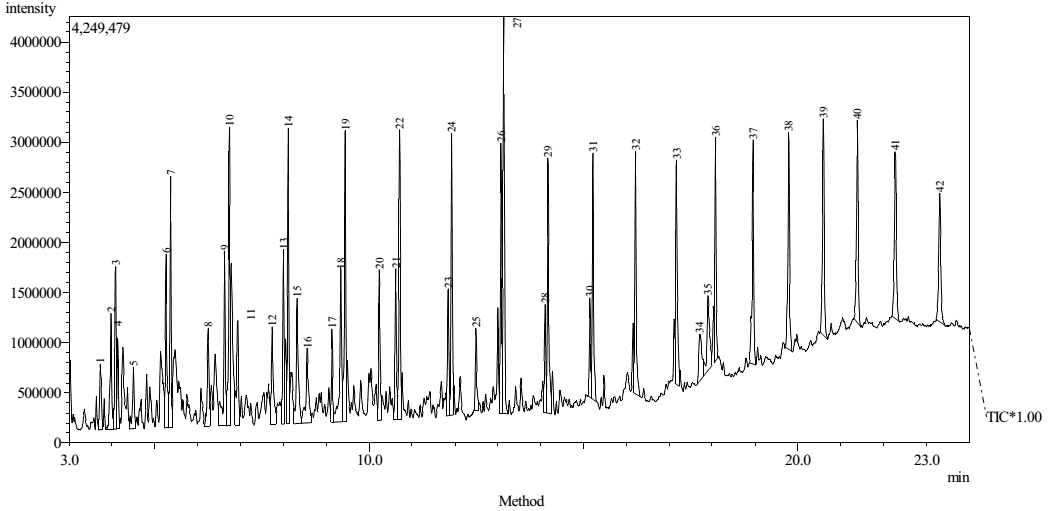 | Figure 1. Chromatogram of Chikila (CHK) coal tar |
|
|
|
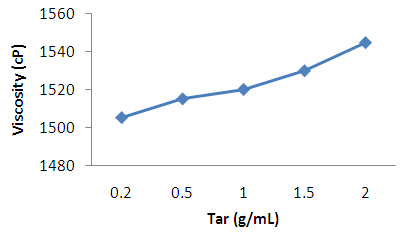 | Figure 2. Effect of tar concentration on the viscosity of shampoo |
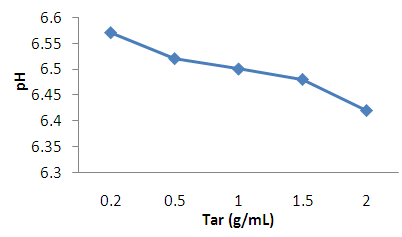 | Figure 3. Effect of concentration on pH of the shampoo |
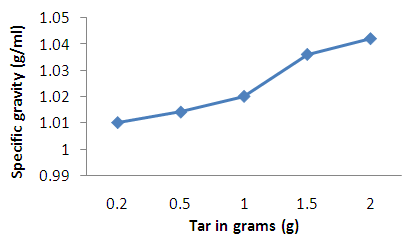 | Figure 4. Effect of concentration on specific gravity of coal tar shampoo |
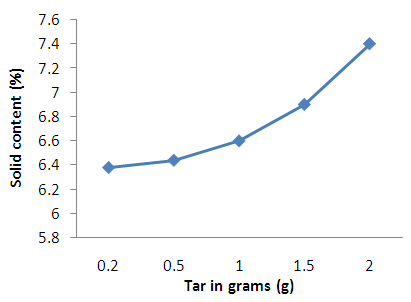 | Figure 5. Effect tar concentration on solid content of shampoo |
4. Conclusions
- The extraction of coal tar from the coal deposit in Chikila, Nigeria, is feasible. The extraction could also be of great economic benefits as the tar yield (46.31%) from the coal is relatively high and encouraging. Some properties of the tar extracted were reported and these will give a more insightful knowledge of its potentials. The quality of the tar reveals its component chemicals some of which are significant in amount and if isolated, will find a wide range of valuable utilites (including pharmaceuticals). The tar extracted was further proven to be of economic values as it was used as a raw material in the optimized production of an industrial product (shampoo). The tailored production of shampoo was aimed at getting the optimum amount of the tar extracted that will be used to produce a product with quality and properties complying with the standards. The amount of the tar extracted within the range 0.5 g – 1.5 g (0.5 – 1.5% respectively) was reported as the optimum for the standard production of shampoo, hence presenting its potential industrial application.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML