J. A. Davlatshoeva, G. B. Eshova, M. M. Rahimova, M. O. Guriev, L. V. Kvyatkovskaya
The Research Institute of the Tajik National University, Rudaki Aven., Dushanbe, Republic of Tajikistan
Correspondence to: M. M. Rahimova, The Research Institute of the Tajik National University, Rudaki Aven., Dushanbe, Republic of Tajikistan.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Complexation in the Fe(II)-Fe(III)-Glycine-Na(H)ClO4-H2O system at a temperature of 298.16 K, the ionic strength of the solution 0.1 ÷ 1.0 (NaC104), CFe (III) = 0, was studied by the Clark-Nikolsky oxidation potential method СFe(III)=СFe(II)=1·10-3 and CGly=1·10-3÷3·10-3 mol/l in the range of pH 0.5-8.0. Experimental curves for the dependence of the emf of the system on the concentration parameters are obtained: рН, pCFe(III), pCFe(II), рСL. To calculate the formation constants of the complexes, the method of successive approximation of the theoretical and experimental oxidative functions using the Excel program was used. The dependence of the formation constant of the complexes on the ionic strength of the solution was calculated on the basis of the Debye-Hückel equation in the program SigmaPlot-10.0. It is established that as the ionic strength of the solution increases, the formation constants of the forming coordination compounds decrease.
Keywords:
Glycinate complexes, Coordination compound formation constant, Ionic strength, Oxredmetry method, Oxidative function, Debye-Hückel equation
Cite this paper: J. A. Davlatshoeva, G. B. Eshova, M. M. Rahimova, M. O. Guriev, L. V. Kvyatkovskaya, Processes of Formation of Glycinate Complexes of Iron (II) and Iron (III) Under Various Ional Forces of Solution, American Journal of Chemistry, Vol. 7 No. 2, 2017, pp. 58-65. doi: 10.5923/j.chemistry.20170702.03.
1. Introduction
It is known that glycine is a bidentate ligand, it can coordinate over amino and carboxyl groups and form homonuclear, binuclear and heteronuclear complexes in solutions with metal ions. A study of the protolytic properties of glycine under experimental conditions for the formation of coordination compounds and the construction of a diagram of the distribution of ionic forms of amino acids show that the cation form of glycine (NH3CH2COOH)+ is present in the solution only in admixture with a bipolar ion (NH3CH2COO) ± for any pH <4.0, > 4.0 it exists in the form of a bipolar ion, and at pH> 9.0 - as an anion (NH2CH2COO)- [1-3].Potentiometric method, at an ionic strength of 0.01 mol/l, the formation of complexes of the composition [FeL] + and [FeL2]0 (where L -glycinate ion) [4] is established. The stepwise instability constants are calculated to be 5.0•10-5 and 3.0•10-4, respectively. In work [5] for the FeII-FeIII-Gly system at an ionic strength of 0.5 mol/l and in the pH range 1.4-10.6, СFe(II)=СFe(III)=1∙10-4; СGly=1∙10-2 mol/l shows the formation of glycinate complexes of iron (II): FeHL(H2O)52+, (lgβ=-0.70±0.04), FeL(H2O)4+, (lgβ=6.00±0.02), Fe2(L)4(OH)2(H2O)62- (lgβ=3.98±0.02), Fe(OH)(H2O)5+ (lgβ=-9.50±0.03).In [6], when studying the role of complex formation and impulse current in the process of electrochemical doping of zinc galvanic coatings with chromium in the zinc (II) -chromium (III) -glycine-water system, it was noted that zinc (II) ions coordinate in the test solution glycinate Ions not only through the amino group, but also the carboxyl group, forming chelate complexes.It was shown in [7] that when glycinate complexes of ferric iron are formed at an ionic strength of 1.0 mol/l and a temperature of 250°C, the following composition is formed: Fe(HL±)3+ (lgβ=1.47), Fe(HL±)23+ (lgβ=3.49), Fe(HL±)A2+ (lgβ=0.32).The purpose of this work is to study the complexation processes in the Fe (II) -Fe (III) -glycine-Na (H) ClO4-H2O system at a temperature of 298.16 K and ionic strengths of the solution 0.1 ÷ 1.0 mol/l. The regularity of the dependence of the stability constants of complexes on ionic strength can be established from the change in the activity coefficients of coexisting ions in solution [8].
2. Experimental
During the experiments, iron (III) and iron (II) perchlorates were used, the initial concentrations of which were determined by trilonometric and bichromatometric methods, respectively [9, 10]. The concentration of the prepared equimolecular mixture of iron perchlorates in two oxidation states was confirmed by a bichromatometric method with preliminary reduction of iron (III) to iron (II) using a Johnson reducer [11]. The concentration of NaClO4 by the previously purified recrystallization was determined by the weight method [12, 13]. Chloric acid HClO4 grade "hp" was used without pre-treatment. The concentration of sodium hydroxide was determined by direct titration with a 0.1 M hydrochloric acid solution of HCl from ficsonal [11, 14].The method of oksredmetry [9, 15] allows the removal of the experimental dependences of the electromotive force (E,mv) on the following concentration variables: рН(-lgh), pCFe(III)(-lgCFe(III)), pCFe(II)(-lgCFe(II)) and рСL(-lgСНL),, where h is the activity of hydrogen ions, and CHL is the concentration of glycine. The experimental procedure consists in measuring the EMF of two galvanic cells I and II.Pt/Fe2+, Fe3+, Gly, H2O//Cl-, AgCl /Ag(I)Pt/Fe2+, Fe3+, Gly, H2O/glass/0,1н НCl/ AgCl /Ag(II)Measurements of the EMF of the galvanic cells were carried out on an EV-74 ionomer with an accuracy of ±1 mV. The pH value in the solutions under study was controlled by a glass electrode from the calibration curve, compiled from the pH values of standard buffer solutions with an accuracy of ±0.05 pH units. The potentials of the silver chloride electrode and the value ν = 2.303RT / F at 298.16 K were taken from the reference book [10].
3. Results and Discussion
The complexation of iron (II) and iron (III) with all forms of glycine, taking into account hydrolytic processes, can be represented as: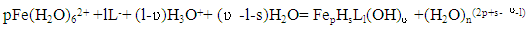 | (1) |
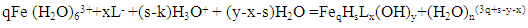 | (2) |
where: p - nuclearity of the complex compound of iron (II); l is the number of ligands; υ is the number of coordinated OH groups; s is the number of protonated groups in the complex, q is the nuclearity of the iron (III) complexes, and k is the number of coordinated OH groups. These particles are basic, since they all co-exist in the system under study and influence each other.According to the theory of the method of oksredmetry, the experimental dependences of the EMF (E,mV) on the pH of the solution in the range of ionic forces 0.1 were obtained to determine the expected composition of the coordination compounds formed in the system under study, as well as the values of the basis particles (q, p, s, l, k) ÷ 1.0 mol/l and different concentrations of iron, which are presented in Figures 1 and 2.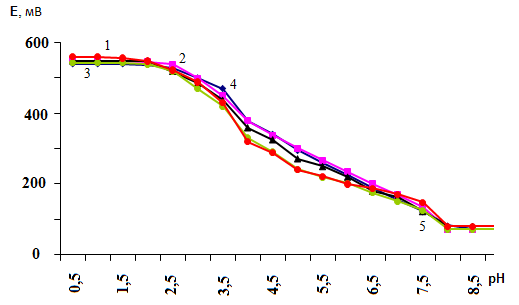 | Figure 1. Dependence of EMF on pH in the system: Fe(II)-Fe(III)-Glycine-Na (H) ClO4-H2O at 298.16 K and various values of the ionic strength of the solution. СFe(III)=СFe(II)=1·10-3 and CGly=1·10-3 mol/l. The curves refer to the ionic strength of the solution (mol / l): 1 -1.00; 2 -0.25; 3 -0.75; 4 -0.10; 5 -0.50 |
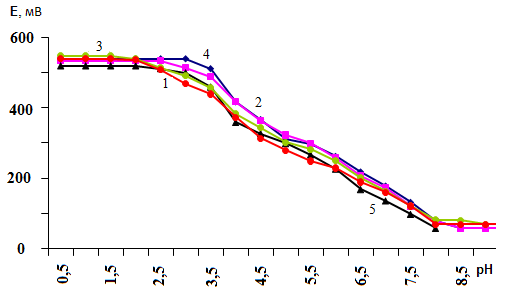 | Figure 2. Dependence of EMF on pH in the system: Fe(II)-Fe (III)-Glycine-Na(H) ClO4-H2O at 298.16 K and various values of the ionic strength of the solution. СFe(III) =СFe(II)=1·10-4 and CGly=1·10-3 mol/l. The curves refer to the ionic strength of the solution (mol/l): 1 -1.00; 2 -0.25; 3 -0.75; 4 -0.10; 5 -0.50 |
The shape of the obtained curves indicates that in the acidic region to pH 3.0, the EMF does not change, hence, the complexation of iron is absent, then, with an increase in pH, it begins and goes to pH 8.5.The concentration of complexing ions depends on the ionic strength of the solution. The lower the ionic strength, naturally, the concentration of complexing ions is lower. Therefore, at lower ionic forces, the onset of complexation shifts toward an increase in pH. For example, at ionic forces of 0.1 and 0.25 mol/l (СFe(III)=СFe(II)=1·10-3 and CGly=1·10-3 mol/l), complexation is absent until pH 2.5, and at ionic forces 0.5, 0.75 and 1.0 mol / l to pH 2.0. At СFe(III) = СFe(II) = 1·10-4 and CGly =1·10-3 and ionic forces 0.1 and 0.25 mol / l - up to pH 3.0, and at ionic forces 0.5; 0.75 and 1.0 mol/l to pH 2.0.Experimental dependences of E on pH were obtained for CFe(II)=CFe(III) =1•10-3; CGly=3•10-3 and an ionic strength of 0.25 mol/l (Figure 3). According to the theory of the method of oksredmetry, the successive formation of linear sections with tangents of the slope angles equal to 0,-ν, -2ν, -3ν, -2ν, -ν, 0 indicates the stepwise complexation of Fe (III) and Fe (II). The E-pH dependences allow one to determine the total number of coordinated ligands around the central ion of the complexing agent.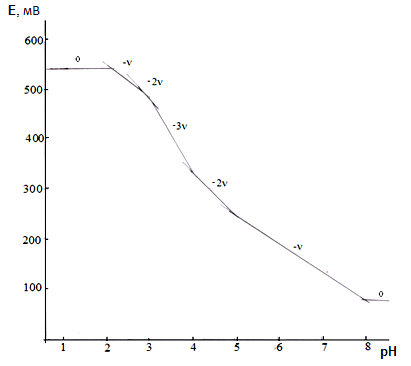 | Figure 3. Dependence of EMF (E, mV) on pH for the Fe(II)-Fe(III)-Glu-H2O system with CFe(II)=CFe(III) =1•10-3, CGly=3•10-3 and I=0.25mol / l |
Experimental curves for the dependence of EMF on other concentration parameters (рСох, рСred, pСL), which have a rectilinear character with a definite slope, are obtained. As an example, we give the dependence of EMF on pCox (Fig. 4).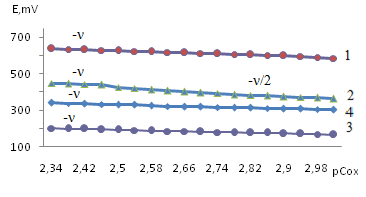 | Figure 4. Dependence of the EMF (E, mV) on pCFe (III) for the Fe(II)-Fe(III)-Glu-H2O system at T = 298.16 K; I = 0.1 and CGly = 1 × 10-3 mol/l. The curves refer to pH: 1-3.0; 2-4.5; 3-6.0; 4-8.0 |
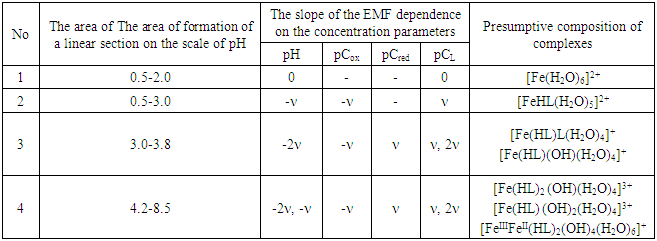
As follows from Figure 4, the curve (2) of the dependence E-pCox consists of two rectilinear sections with tangents of the slope angle -ν and -ν/2, and the others are characterized by one linear section with an angular coefficient equal to -ν. According to the theory of the method, this indicates the formation of mono- and binuclear coordination compounds Fe(III) in the solutions under study.Analysis of the slopes of all the experimental curves obtained made it possible to construct a matrix of values of the angular coefficients [16], with the help of which the expected compositions of the resulting coordination compounds were determined (Tables 1, 2).Table 1. The experimental values of the angular coefficients of the EMF dependences on the concentration variables of the Fe(II)-Fe(III)-glycine-water system for Fe(II) complexes at 298.16 K, I=0.5; CFe(II)=CFe(III) =1•10-3 and СGly=1•10-3 mol/l
 |
| |
|
Table 2. The experimental values of the angular coefficients of the EMF dependences on the concentration variables of the Fe(II)-Fe(III)-glycine-water system for Fe(III) complexes at 298.16 K, I =0.5; CFe(II)=CFe(III) =1•10-3 and СGly=1•10-3 mol/l
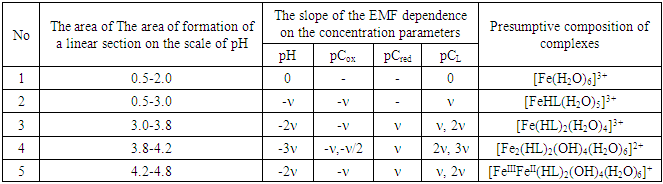 |
| |
|
To calculate the equilibrium constants, Yusupov's oxidizing function was used [17]. Based on the experimental values of the EMF on its dependence on pH, the values of the experimental oxidizing function E were first calculated from the equation: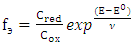 | (3) |
where: E is the experimentally measured value of EDS, E0 is the value of the standard EDS, and a = 2.303 RT/F. The theoretical value of ƒт was calculated taking into account the established compositions of coordination compounds according to the following equations: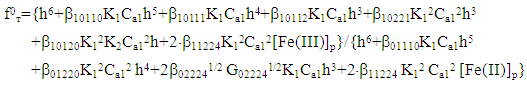 | (4) |
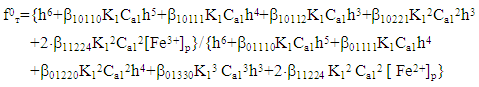 | (5) |
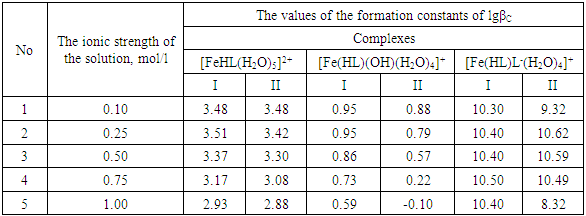
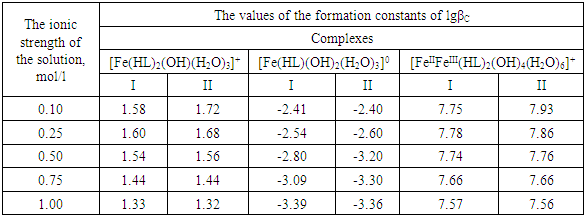
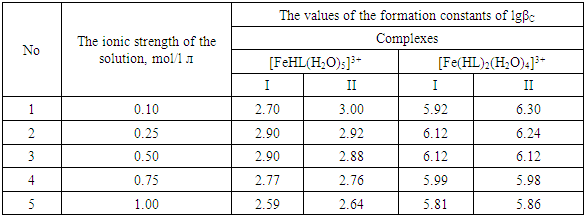
Using these equations, stability constants of coordination compounds of the following composition were determined for all studied concentrations of iron (II), iron (III) and glycine: FeHL(H2O)52+, [Fe(HL)L-(H2O)4]+, Fe(HL)(OH)(H2O)4+, Fe(HL)(OH)2(H2O)30, Fe(HL)2(OH)(H2O)3+ - for ferrous iron and FeHL(H2O)53+, Fe(HL)2(H2O)43+, Fe2(HL)2(OH)4(H2O)62+, Fe(HL)(OH)(H2O)42+, Fe(HL)3(H2O)33+, FeIIIFeII(HL)2(OH)4(H2O)6+ - for ferric iron. From these equations it follows that in the system iron (II) - iron (III) - Gly - H2O at СFe(III)=СFe(II)=1·10-3, СGly=1∙10-3÷3∙10-3 binuclear complexes Fe2III(HL)2(OH)4(H2O)62+ and [FeII(HL)L-(H2O)4]+ are formed, and for Cgly = 1 ∙ 10-4 mol/l instead of these Particles, coordination compounds of the composition FeIII(HL)3(H2O)33+ and FeIII(HL)(OH)(H2O)42+ are formed.Further, by the method of iteration of the experimental oxidizing function with the theoretical, deduced by us taking into account the composition of the formed coordination compounds, their formation constants are calculated (Tables 3 and 4).Table 3. Numerical value of the stability constants of Fe(II) complexes in iron (II)-iron (III)-Gly-H2O systems at 298.16 K and various ionic strengths of the solution, СFe(III)=СFe(II)=1·10-3; СGly=1∙10-3 mol/l
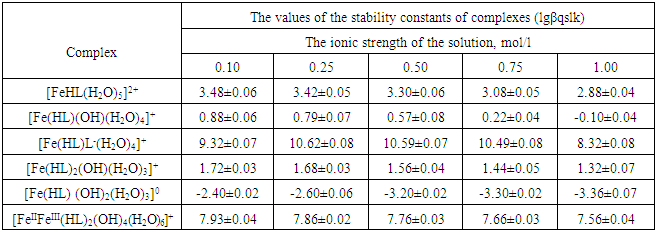 |
| |
|
Table 4. Numerical values of the stability constants of Fe (III) complexes of iron(II)-iron(III)-Gly-H2O system at 298.16 K, СFe(III)= СFe(II)=1·10-3; СGly=1∙10-3 mol/l and various ionic strengths of the solution
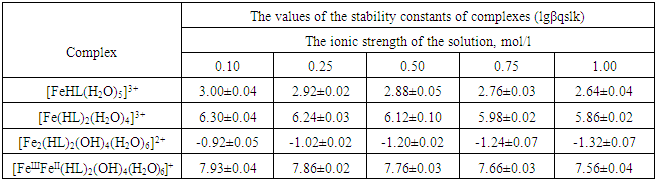 |
| |
|
It is established that the ionic strength of a solution affects the numerical values of the constants of the formation of coordination particles. When the ionic strength is increased from 0.1 to 1.0 mole/liter, the logarithms of the formation constants (lgβqslk) of the complexes (Tables 3-4) gradually decrease, which is associated with a change in the activity coefficients, therefore, the concentration-dependent formation constants also change [8].In the Fe(II)-Fe(III)-Gly-H2O system for Fe(III) complexes at the concentration parameters CFe(II)=CFe(III) =1.10-3, CGly=1•10-3, 2•10-3, 3•10-3 mol/l, 4 complexes of the following composition are formed: FeHL(H2O)53+, Fe(HL)2(H2O)43+, Fe2(HL)2(OH)4(H2O)62+, FeIIIFeII(HL)2(OH)4(H2O)6+.With the increase in the ratio of the ligand (CFe(II)=CFe(III)=1.10-4 and CGly=1•10-3, 2•10-3, 3•10-3 mol/l), two others are formed instead of the binuclear complex: Fe(HL)(OH)(H2O)42+ and Fe(HL)3(H2O)33+, since the ligand concentration increases.With increasing pH and glycine concentration, the stability of the resulting mononuclear complexes increases, for example, FeHL(H2O)53+ (lgβ=2.82±0.02); Fe(HL)2(H2O)43+ (lgβ=5.58±0.04) and Fe(HL)3(H2O)33+ (lgβ=7.02±0.02). With a change in pH toward the alkaline region, the concentration of ligands HL increases, which leads to the formation of more saturated coordination compounds. The same effect is manifested when the concentration of glycine increases. The more ligands in the inner sphere of the complex, the greater its stability.It is known that the dependence of the concentration equilibrium constant of coordination compounds on the ionic strength of the solution is expressed by one of the equations of the Debye-Hückel theory [18]: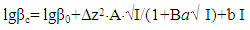 | (6) |
where: A and B are constants, for aqueous solutions at 25°C, A = 0.509 and B = 0.328; Δz2 is the algebraic sum of the squares of the ion charges, I is the ionic strength of the solution, b is the empirical constant characterizing the change in the dielectric constant of the medium near the ion; A-variable parameter equal to the average effective diameter of the hydrated ion, expressed in angstroms (а = 0.49 nm = 4.9 Angstroms), β0 is the thermodynamic constant, βC is the concentration constant of complex formation.On the basis of equation (6), a dependence is constructed on the ionic strength (I) of the solution (Figures 5, 6, a). The resulting straight lines, when extrapolated to I = 0, give the segments on the ordinate axis numerically equal to lgβ0. The angular coefficient of the obtained curves made it possible to determine the value of the empirical constant b of equation (6), which are given in Table 5.Table 5. The thermodynamic (lgβ0) and empirical (b) constants for Fe(II) complexes formed in the Fe(II)-Fe(III)-glycine-Na(H)ClO4-H2O system at 298.16K, СFe(III)=СFe(II)=1·10-3 and CGly =1·10-3 mol/l
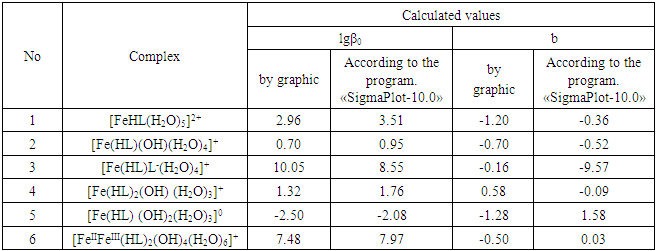 |
| |
|
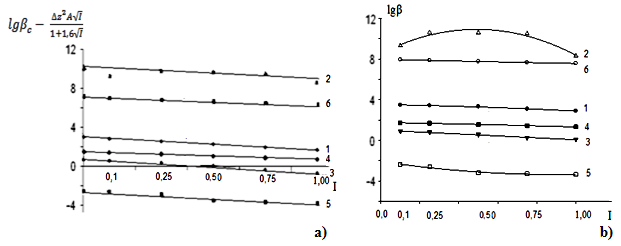 | Figure 5. Dependence of the formation constants of complex compounds Fe (II) on the ionic strength of solution (I) mol l in the system: Fe (II) -Fe (III) -glycine-Na (H) ClO4-H2O, 298.16 K, СFe(III)=СFe(II)=1·10-3 and CGly =1·10-3 mol/l. The curves refer to complex compounds: 1-FeHL(H2O)52+, 2-[Fe(HL)L-(H2O)4]+, 3-Fe(HL)(OH)(H2O)4+, 4-Fe(HL)2(OH)(H2O)3+, 5-Fe(HL)(OH)2(H2O)30, 6-FeIIFeIII(HL)2(OH)4(H2O)6+; a) by the Debye-Hückel equation; b) under the program "SigmaPlot-10.0" |
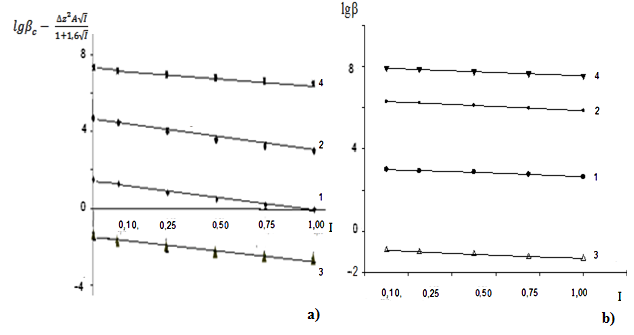 | Figure 6. Dependence of the formation constants of Fe (III) complexes on the ionic strength of solution (I) mol/l in the system: Fe(II)-Fe(III)-glycine-Na(H)ClO4-H2O, 298.16 K, СFe(III)=СFe(II)=1·10-3 and CGly =1·10-3 mol/l. The curves refer to complex compounds: 1-FeHL(H2O)53+, 2-Fe(HL)2(H2O)43+, 3-Fe2(HL)2(OH)4(H2O)62+, 4-FeIIIFeII(HL)2(OH)4(H2O)6+; a) by the Debye-Hückel equation; b)under the program "SigmaPlot-10.0" |
In the studied system, the calculated values of the empirical constant (b) take negative values for all the complexes formed (except for Fe(HL)2(OH)(H2O)3+), so equation (6) takes the following form: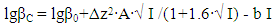 | (7) |
Taking into account the calculated values of the constants, the following equations correspond to the complex compounds formed according to equation (6):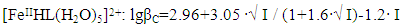 | (8) |
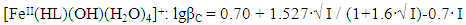 | (9) |
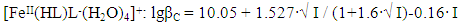 | (10) |
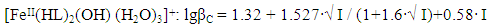 | (11) |
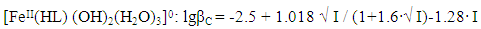 | (12) |
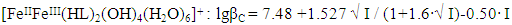 | (13) |
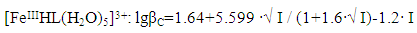 | (14) |
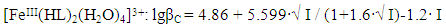 | (15) |
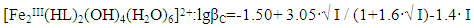 | (16) |
It follows from Eqs. (8-16) that the quantity representing the interionic distance (A = 0.509) remains constant, Δz2 varies depending on the charge of the complex particle, and these equations differ in the values of the thermodynamic constants (lgβ0) of complex formation and the empirical constant (b).Calculations and statistical processing of constants were carried out using the program "SigmaPlot-10.0" (Fig. 5b, 6b, Table 5). The obtained dependences are described by the equation: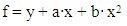 | (17) |
Values of the logarithm of the thermodynamic constants (lgβ0) of the resulting coordination compounds obtained by the two methods (Table 5) are consistent. The values of the constants of the third complex [Fe (HL)L- (H2O)4]+ differ from each other, which can be explained by the extreme nature of the curve as a function of the ionic strength (I) of the solution, i.e. First there is an increase in the curve, which reaches a maximum to an ionic strength of 0.5 mol/l, then a decrease to an ionic strength of 1.0 mol/l. The values of the empirical constant (b) of equations (8-16) are consistent among themselves within the error of the calculation method.The constants of the formation of coordination compounds, calculated from the experimental data obtained by the iteration method and calculated with the help of the corresponding equations (8-17), and also using the program "SigmaPlot-10.0" are in good agreement (Tables 6, 7, 8, 9), which indicates the reliability of the results obtained.Table 6. The values of the concentration constants for the formation of Fe(II) complexes are of the following composition: FeHL(H2O)52+, Fe(HL)(OH)(H2O)4+, [Fe(HL)L-(H2O)4]+ of System: Fe(II)-Fe(III)-glycine-Na(H)ClO4-H2O at 298.16 K, СFe(III) =СFe(II)=1·10-3 and CGly=1·10-3 mol/l (I - calculated by the equation, II - found experimentally)
 |
| |
|
Table 7. The values of the concentration constants for the formation of Fe(II) complexes are of the following composition: FeHL(H2O)52+, Fe(HL)(OH)(H2O)4+, [Fe(HL)L-(H2O)4]+ of System: Fe(II)-Fe(III)-glycine-Na(H)ClO4-H2O at 298.16 K, СFe(III) =СFe(II)=1·10-3 and CGly=1·10-3 mol/l (I - calculated by the equation, II - found experimentally)
 |
| |
|
Table 8. The values of the concentration constants for the formation of Fe(III) complexes of the composition: FeHL(H2O)53+, Fe(HL)2(H2O)43+ of System: Fe(II) -Fe(III)-glycine-Na(H)ClO4-H2O at 298.16 K, СFe(III)=СFe(II)=1·10-3 and CGly=1·10-3 mol/l (I-calculated from the equation, II-found experimentally)
 |
| |
|
Table 9. The values of the concentration constants for the formation of Fe(III) complexes are of the following composition: Fe2(HL)2(OH)4(H2O)62+, FeIIIFeII(HL)2(OH)4(H2O)6+ of System: Fe(II )-Fe(III)-glycine-Na(H)ClO4-H2O at 298.16 K, СFe(III) =СFe(II)=1·10-3 and CGly =1·10-3 mol/l (I-calculated according to the equation, II-found experimentally)
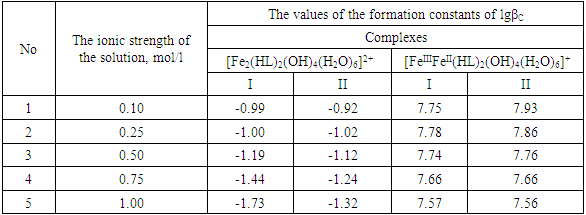 |
| |
|
4. Conclusions
Thus, the complexation in the Fe(II)-Fe(III)-Glycine-Na(H)ClO4-H2O system at 298.16 K, the ionic strength of the 0.1 ÷ 1.0 (NaC104) solution in the pH range 0.5-8.0 was studied by the oxidation potential method. By the method of iteration of the experimental oxidizing function with the theoretical according to the Excel program, the compounds and regions of existence of complex compounds found by the oxidation potential method were confirmed and the formation of complex compounds not achieving dominance was established. The regions of their existence are determined by pH and concentration constants of formation are calculated. The thermodynamic formation constants are calculated using the corresponding Debye-Hückel equations and using the program "SigmaPlot-10.0". The constants of the formation of coordination compounds are in good agreement with each other, which indicates the reliability of the results obtained. From the data of the formation constant, it follows that as the ionic strength of the solution increases, the formation constants of the complex compounds formed decrease.
References
| [1] | Yusupov Z.N., Eshova G.B., Saidov S. S. The influence of ionic strength on the ionization constant of aminoethanoic acid. J. Reports of the Academy of Sciences of the Republic of Tajikistan. 2008. Vol.51, No 8. 620-625. |
| [2] | Kvyatkovskaya L.V., Eshova G.B., Rahimova M.M., Davlatshoeva D. A. Investigation of complex formation in the iron (II) -glycine-water system at an ionic strength of 1.0 mol/l. Bulletin of Tajik National University. A series of natural sciences.. Dushanbe. 2014. No.1/4 (153) 86-95. |
| [3] | Albert A., Sergeant E., The constants of ionization of acids and bases. Moscow. Chemistry, 1964. 178. |
| [4] | Handbook of the chemist (ed. Nikolsky B.P., Grigorova O.N.). Moscow. Chemistry, 1965. 1005. |
| [5] | Rahimova M.M. Complexation of Fe, Co, Mn and Cu ions with mono- and polybasic organic acids, neutral ligands in aqueous solutions: dissert. Doct. Chem. Sciences. Dushanbe, 2013. |
| [6] | Berezin N.B., Filippova A.G., et al. Electrochemical doping of zinc coatings with chromium from electrolytes based on heteronuclear complexes. J. Chemistry and Computational Simulation. Butlerov messages. 2004. Vol.5, No. 1. 39 -43. |
| [7] | Yakubov H.M., Shcherbakova V.I., Palchevsky V.V., Bukharizada R.A. Glycinate complexes of iron. Reports of the Academy of Sciences of the Tajik SSR. 1975. Vol. XVIII, No.4. 36 - 38. |
| [8] | Beck M., Nagpal I. Investigation of complexation by the latest methods. Moscow. The World, 1989. 405. |
| [9] | Yakubov H. M. Application of oxredmetry to the study of complex formation. Dushanbe. Donish, 1966. |
| [10] | Mishchenko K.P., Ravdel A. A. A brief reference book of physicochemical quantities. Leningrad. Chemistry, 1974. |
| [11] | Suslennikova V. M., Kiseleva E.K. A guide to the preparation of titrated solutions. Leningrad. Chemistry, 1968. 45 – 71. |
| [12] | Volumetric analysis (Koltgof I. M., Belger R., Stenger VA. A., Matsuyama J.). Moscow. Goskhimizdat, 1961. Vol.3. |
| [13] | Charlot G. Methods of Analytical Chemistry. Quantitative analysis of inorganic compounds. trsl from fr. Lurie Yu. Yu. Moscow. Chemistry, 1965. P. 930. |
| [14] | Korostelev P.P. Preparation of solutions for chemical-analytical work. Moscow. Academy of Sciences of USSR, 1962. |
| [15] | Nikolsky B.P., Palchevsky V.V., Pendin A.A., Yakubov H. M. Oxredmetry. Leningrad. Chemistry, 1975. |
| [16] | Eshova G.B., Davlatshoeva J.A., Rakhimova M.M., Kvyatkovskaya L.V. Investigation of complex formation in iron (II) -iron (III) -glycine-water system at an ionic strength of 0.10 mol/l. Herald of Tajik National University. Series of natural sciences 1/4 (216). Dushanbe: "Sino". 2016. 235 - 241. |
| [17] | Pat. TJ 295, Republic of Tajikistan, (51) 7 G 01 N 27/26, C 25 B 3/12. Method for determining the composition and the constants of the formation of coordination compounds (Yusupov Z.N.). The applicant and the patent holder are Tajik State National University. - No. 97000501; Application form 16.12.1997). It is registered in Bull. No. 21. 21.12.2000. |
| [18] | Vasilyev V.P. Thermodynamic properties of electrolyte solutions. Moscow: Higher School, 1982. |


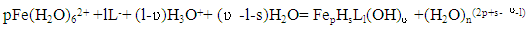
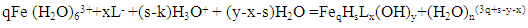


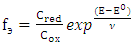
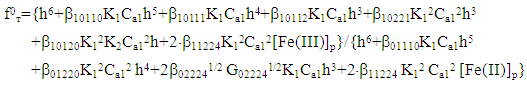
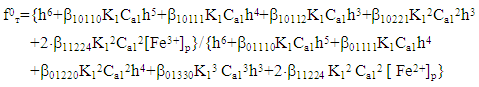
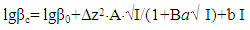


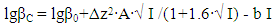
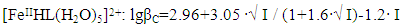
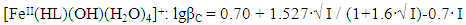
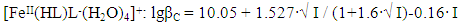
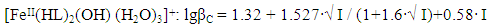
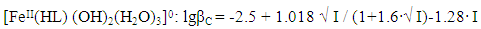
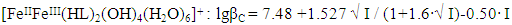
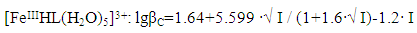
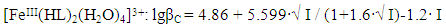
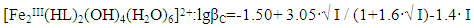
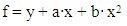
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML









