-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2017; 7(2): 21-47
doi:10.5923/j.chemistry.20170702.01

The Six Silent Laws of Chemical Clusters
Enos Masheija Rwantale Kiremire
Department of Chemistry and Biochemistry, University of Namibia, Private Bag, Windhoek, Namibia
Correspondence to: Enos Masheija Rwantale Kiremire, Department of Chemistry and Biochemistry, University of Namibia, Private Bag, Windhoek, Namibia.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

During careful study of chemical clusters over several years with the help of 14n/4n series independently developed here, it was discerned that the clusters portray outstanding, persistent and highly orderly features. In their formation, they follow certain strict mathematic rules. Six important principles which prominently stand out have been identified. These are –the 14n/4n rule, skeletal linkage values of cluster elements, the conservation of skeletal linkage values, the cluster parameter K(n) linkage with skeletal symmetry, the K(n) correlation series and the Cluster Chain Length. Also by analyzing Capping series, a concept of ‘CLUSTER HOLE FORMALISM’ was introduced. A cluster table previously derived was found to be exceedingly useful in categorizing a wide and large range of clusters using K(n) values calculated from skeletal linkage values. A long table of categorized chemical clusters is provided. Additional interesting examples demonstrate the rapid ease of application of the table and K(n) values to categorize the chemical clusters, and in some cases predict the way they were included towards the end of the paper. This paper sums up the major highlight features of clusters derived through a thorough analysis of many chemical clusters from small ones such as diatomic species to giant ones comprising of fifty or more atoms using the series method.
Keywords: Skeletal-Numbers, Chemical-Law, Hole-Formalism, Clusters, Series, Chain-Length
Cite this paper: Enos Masheija Rwantale Kiremire, The Six Silent Laws of Chemical Clusters, American Journal of Chemistry, Vol. 7 No. 2, 2017, pp. 21-47. doi: 10.5923/j.chemistry.20170702.01.
Article Outline
1. Introduction
- Chemical clusters have fascinated scientists for a number of decades and they continue to do so [1-37]. During the study of chemical clusters, it was observed that the clusters follow consistent natural patterns (laws). The patterns have briefly been touched in previous work [30, 33]. After a careful scrutiny of the work on clusters, it has been found appropriate to clearly spell out these silent and important cluster patterns so that they can freely be utilized by scholars. These findings are summarized in this paper. It is proposed that we refer to them as rules or chemical cluster laws. The purpose of this paper is to briefly explain and highlight these laws.
1.1. Background
1.1.1. Origin of the 14n /4n Rule
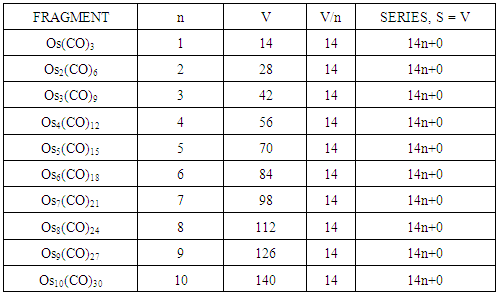
- In an attempt to understand PSEPT concept [1, 2, 8, 10] and to find out if there is some order or disorder among clusters, a systematic study on osmium carbonyl clusters was carried out [32]. What was found interesting was that an osmium fragment, Os(CO)3, with a content of 14 valence electrons [8+3x2] could be utilized as a starting point to generate as many hypothetical osmium clusters of different series as possible [33]. Multiples of the fragment, produced the same series S = V = 14n+0 (S = series, V = valence electrons). This is shown below.
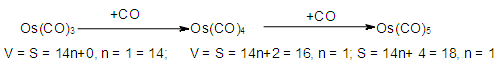 n = the number of skeletal atoms in a cluster.
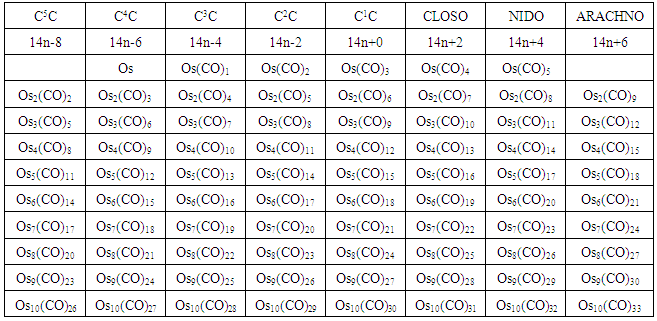
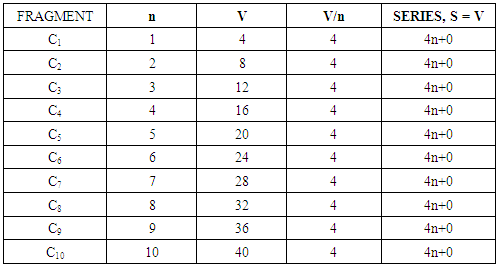
n = the number of skeletal atoms in a cluster.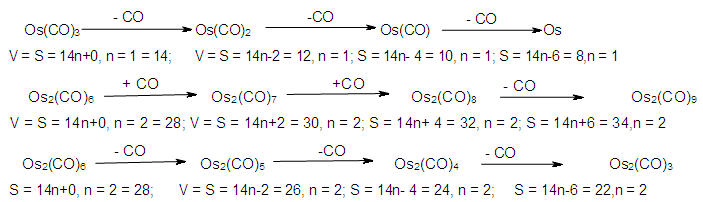 The derivation of selected the hypothetical carbonyl clusters of osmium of CLAN S = 14n+0 series are shown in Table-A and the capping and decapping series also generated using fragment F = Os(CO)3 as a starting point are shown in Table-B. Under the column headed by C1C are the same carbonyl clusters shown in Table-A. Also in Table-B shows a very important diagonal of cluster members Os10(CO)27, Os9(CO)23, Os8(CO)23, Os7(CO)21, Os6(CO)19[Os6(CO)182-, an octahedral complex]. The clusters before the octahedral complex are all capping series of the cluster geometry. The other two clusters in the diagonal namely, Os5(CO)17 and Os4(CO)15, correspond to the NIDO and ARACHNO members of the series. The hypothetical carbonyl clusters of rhenium, rhodium and palladium were similarly generated using the 14-electron fragments, Re(H)(CO)3, Rh(H)(CO)2, and Pd(CO)2 respectively [32-33]. A search for a corresponding fragment of the MAIN GROUP ELEMENTS that could give the series S = 4n+0 was found to be [C] and the corresponding selected series are given in Table-C. These fragments are clearly isolobal, Os(CO)3⥈ Re(H)(CO)3⥈ Rh(H)(CO)2⥈ Pd(CO)2⥈C.
The derivation of selected the hypothetical carbonyl clusters of osmium of CLAN S = 14n+0 series are shown in Table-A and the capping and decapping series also generated using fragment F = Os(CO)3 as a starting point are shown in Table-B. Under the column headed by C1C are the same carbonyl clusters shown in Table-A. Also in Table-B shows a very important diagonal of cluster members Os10(CO)27, Os9(CO)23, Os8(CO)23, Os7(CO)21, Os6(CO)19[Os6(CO)182-, an octahedral complex]. The clusters before the octahedral complex are all capping series of the cluster geometry. The other two clusters in the diagonal namely, Os5(CO)17 and Os4(CO)15, correspond to the NIDO and ARACHNO members of the series. The hypothetical carbonyl clusters of rhenium, rhodium and palladium were similarly generated using the 14-electron fragments, Re(H)(CO)3, Rh(H)(CO)2, and Pd(CO)2 respectively [32-33]. A search for a corresponding fragment of the MAIN GROUP ELEMENTS that could give the series S = 4n+0 was found to be [C] and the corresponding selected series are given in Table-C. These fragments are clearly isolobal, Os(CO)3⥈ Re(H)(CO)3⥈ Rh(H)(CO)2⥈ Pd(CO)2⥈C.
|
|
|
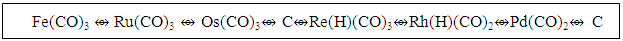 Some of the carbon fragment series are shown below.
Some of the carbon fragment series are shown below.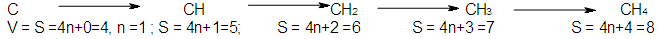
1.1.2. The Origin of the K- Value
- Consider the following operations of fragments and series.CH + CH → C2H2 → H―C≡C―H4n+1(n =1) + 4n+1(n +1) → 4n+2(n + 2); K = 2n-1 = 2(2)-1 = 3CH2 + CH2 → C2H4 → H2C=CH2S = 4n+2(n =1) + 4n+2(n +1) → 4n+4(n + 2); K = 2n-2 = 2(2)-2 = 2For C2H6, S = 4n+6, K = 2n-3 = 2(2)-3 = 1.
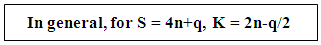
1.1.3. Isolobal Relationship
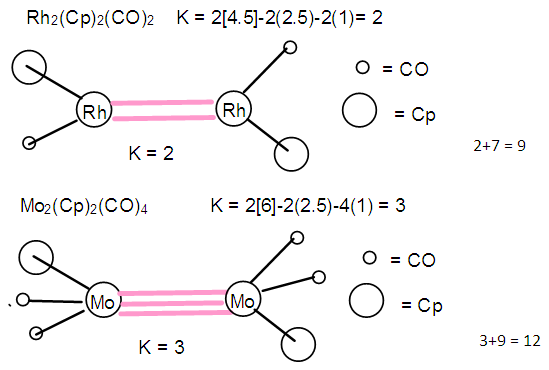
- It was also found from the isolobal relationship [27] that 14n+q series behave in the same way as 4n+q. Furthermore, we could also apply the K = 2n-q/2 relationship to calculate cluster linkages in transition metal clusters. Let us consider Fe2(CO)9, S →2[Fe(CO)3]+3CO→S = 14n+6(n= 2). This series also corresponds to S = 4n+6, hence K = 2n-3 = 2(2)-3 = 1. This means that the complex has one Fe―Fe bond. This cluster behaves in a similar was as C2H6 whose K= 1. Thus, Fe2(CO)9 ⥈ C2H6. If we consider another complex, Rh2(Cp)2(CO)2. This can be fragmented into 2[Rh(Cp)]+ 2CO→S = 14n+4→S =4n+4 and K = 2n-2 = 2(2)-2 = 2. This implies the existence of a double bond, [Rh=Rh] in the cluster [24]. Lastly, the complex, Mo2(Cp)2(CO)4→2[Mo(Cp)(CO)2]→S =14n+2→S = 4n+2 and K = 2n-1 = 2(2)-1 = 3. This predicts the presence of [Rh≡Rh] triple bond in the cluster and this is indeed the case [24]. Furthermore, it was proposed instead of using 14n+q for transition metal clusters for analysis, we could as well, use 4n+q which is simpler except in cases where the value of valence electrons are required. More close studies of the series has recently led to the discovery of the SKELETAL NUMBERS. These have made the analysis and categorization clusters far much faster and easier. A good collection of background references of the origin of the laws is included under the author’s name. With this concise background on cluster series, it is hoped that the sifted cluster laws will be appreciated by our readers.
2. Results and Discussion
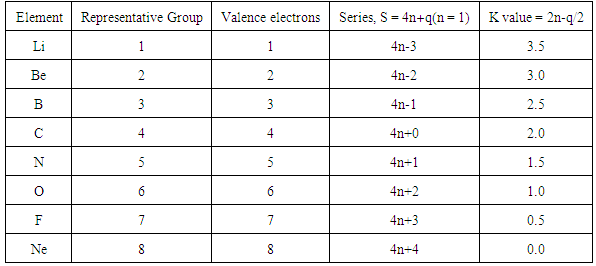
2.1. The First law: The 4n/14n law
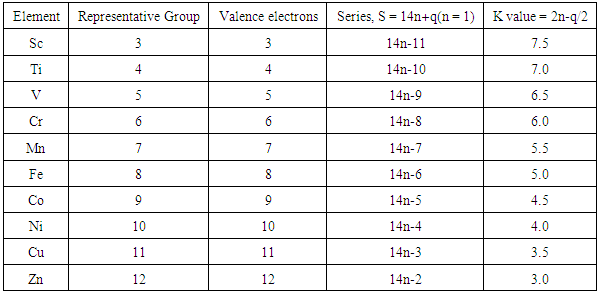
- It has been found that the main group elements follow the 4n law (rule) while the transition metal elements follow the 14n law (rule) of clusters series. The 14n and 4n rules (series) were also used to categorize clusters [27-30]. As a consequence of this, the skeletal numbers which constitute the second law were derived [27-30]. Sample of selected elements are given in Tables 1 and 2 and their origin was discussed in previous work [27-30].
2.2. The Second Law: Skeletal Linkage Numbers and Their Periodicity
|
2.2.1. Skeletal Numbers of Transition Elements (K values)
|
2.3. The Third Law: Conservation of Skeletal Numbers
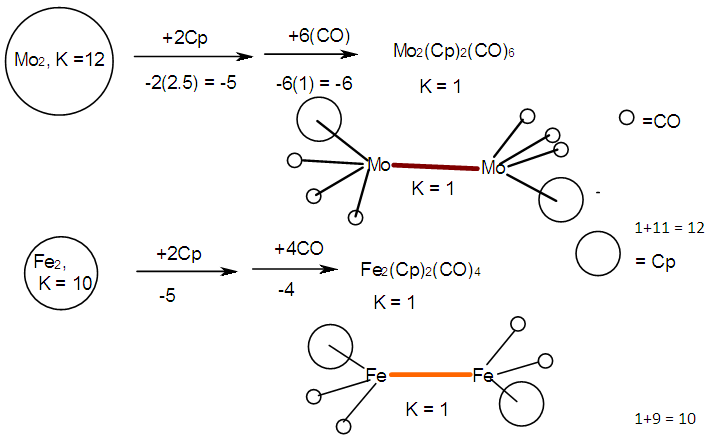
- The conservation of skeletal numbers has already been discussed in previous work [30, 33-34]. In order to re-emphasize the concept, a few examples are given as illustrations in pictorial forms in Schemes 1 to 4.
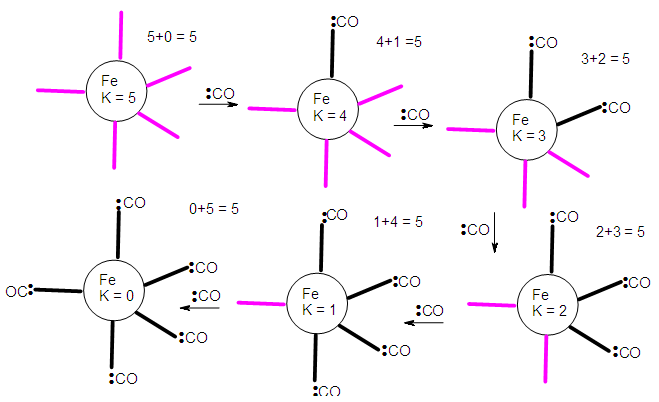 | Scheme 1. Demonstration of the law of conservation of skeletal linkage numbers by Fe fragments |
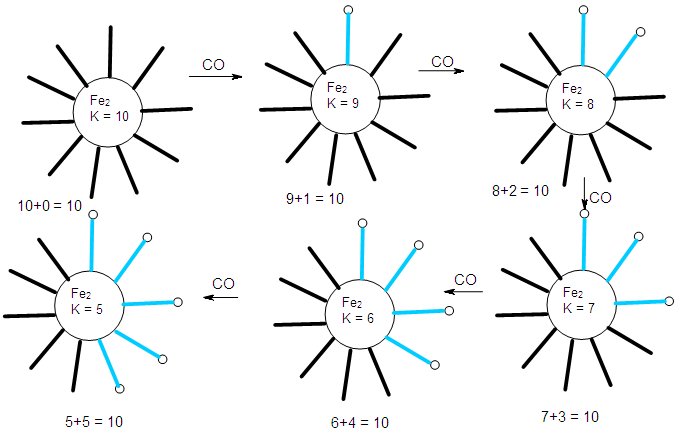 | Scheme 2. Conservation of skeletal linkage numbers of Fe2 cluster and its fragments |
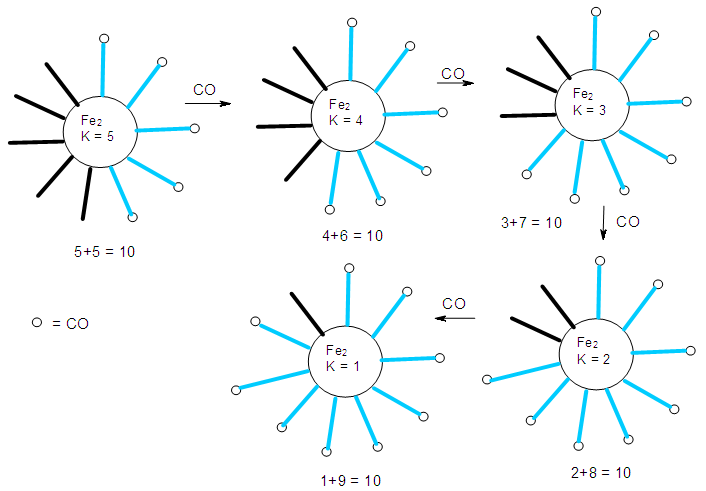 | Scheme 2. Continued |
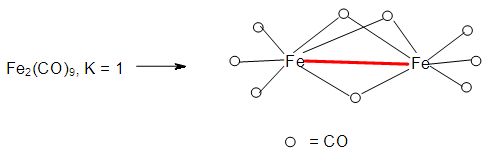 | Figure 1. Possible isomer of Fe2(CO)9 cluster |
 | Scheme 3. More examples to demonstrate the Law of Conservation of Cluster linkages |
 | Scheme 4. More examples on Conservation Law of Skeletal linkage Numbers |
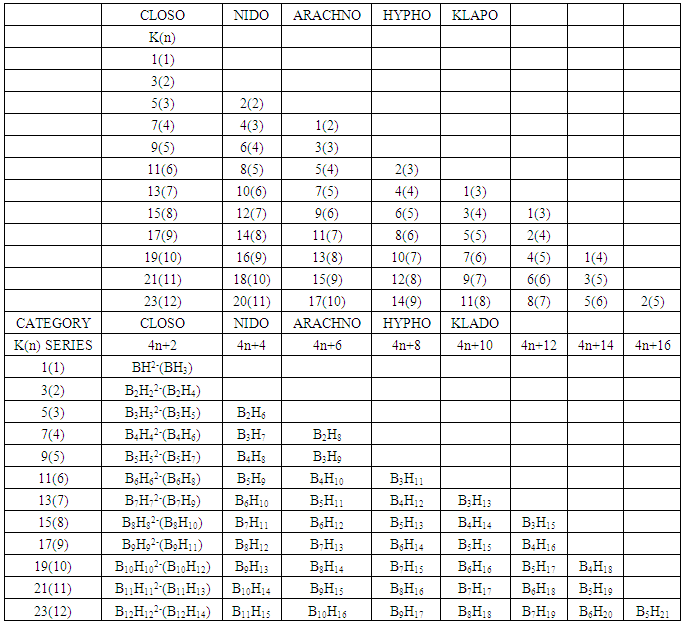
2.4. The Fourth Law: The K(n)-Symmetry Linkage Law
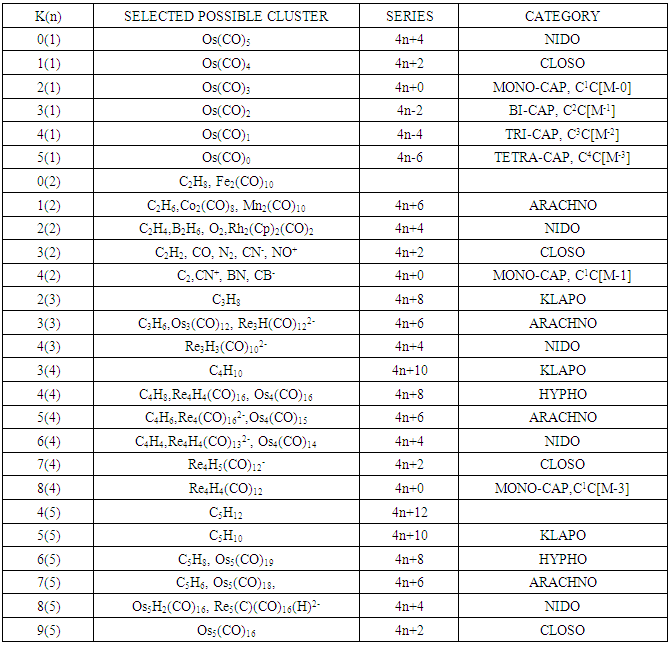
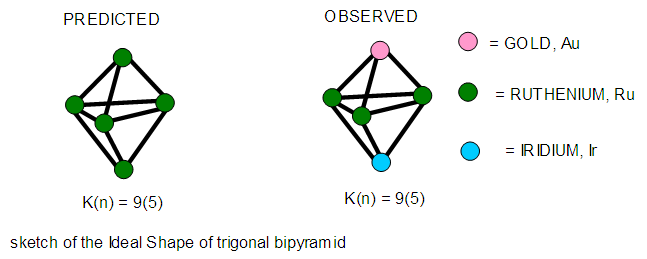
- 2.4.1 This simply means that a given K(n) parameter of a cluster can be associated with a certain skeletal cluster symmetry. The n stands for the number of skeletal elements and K represents the number of skeletal linkages corresponding to the cluster under consideration. For instance K(n) = 1(2), means that there are 2 skeletal elements linked by one bond. Such clusters belong to the series S = 4n+6(ARACHNO) (see Table 4). Examples include [6, 21] Mn2(CO)10, Fe2(CO)9, and C2H6. More examples can be found in Table 3. The K(n) = 2(2), means that 2 skeletal elements are linked by a double bond. It belongs to the series S = 4n+4(NIDO). Examples include, Rh2(Cp)2(CO)2, C2H4, and O2. On the other hand K(n) =3(2) means that 2 skeletal atoms are linked by a triple bond. The clusters belong to the series S = 4n+2(CLOSO). The examples include, C2H2, and Mo2(Cp)2(CO)4. The K(n) = 6(4)→ represents an ideal tetrahedral symmetry. In this case, the cluster has 4 skeletal atoms and 6 linkages. It belongs to the series S = 4n+4(NIDO). Examples [6] include P4, Ir4(CO)12 and InBi32― (for more examples see Table 3). The ideal skeletal tetrahedral geometry for P4 and Ir4(CO)12 is sketched in Figure 5. The parameter K(n) = 8(5) represents 5 skeletal elements and 8 linkages. It is the ideal geometry of a Square pyramid. It belongs to the series S = 4n+4(NIDO). Examples include [5, 6, 14] B5H9, C2B3H7, C5H5+, B3H7(CoCp*)2, Fe5(C)(CO)15, and Ru5(C)(CO)15 (for more examples see Table 3). The trigonal bipyramid geometry is represented by K(n) = 9(5) parameter. That is 5 skeletal elements with 9 linkages. Checking on Table 4 they are found to belong to series S = 4n+2(CLOSO). Examples include [5, 6, 31-32] B5H52―, Sn52―, Bi53+, Te2CrMo2(CO)102― and Os5(CO)152― (for more examples, see Table 3). Figures 2, 3 and 4 show linkage between K(n) and ideal symmetries of selected samples of clusters are given below. Figure 2[(iv)] focuses mainly on an ideal trigonal bipyramid symmetry. The octahedral geometry has code K(n) = 11(6) and is shown in Figure 4. The complexes with the ideal octahedral shape (see Figure 6), among others, include [34-37] Ru6(C)(CO)14(C6H6), Rh6(CO)16, Os6(CO)182-, Re6(C)(CO)192-, Co4Ni2(CO)142-, Ru6(H)(CO)18-, Ru6(C)(CO)18-, TeRu5(CO)142-, HTeRu5(CO)14-, Te2Ru4(CO)11, Se2Mn4(CO)122-, SFe2Ru3(CO)142-, B6H62-, Os6(H)(CO)18-, Re6(C)(CO)19(H)-, Re6(C)(CO)19(H)-, Re6(C)(CO)18(H)3-, Re6(C)(CO)18(H)22- and Re6(H)7(CO)18-.We can regard every K(n) code to represent a particular structural geometrical shape or its corresponding isomer.
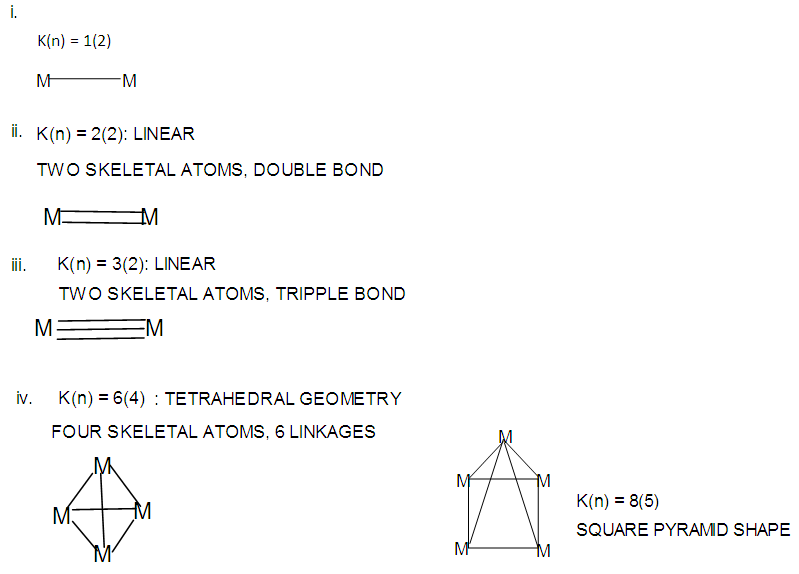 | Figure 2. Various geometries of selected skeletal clusters |
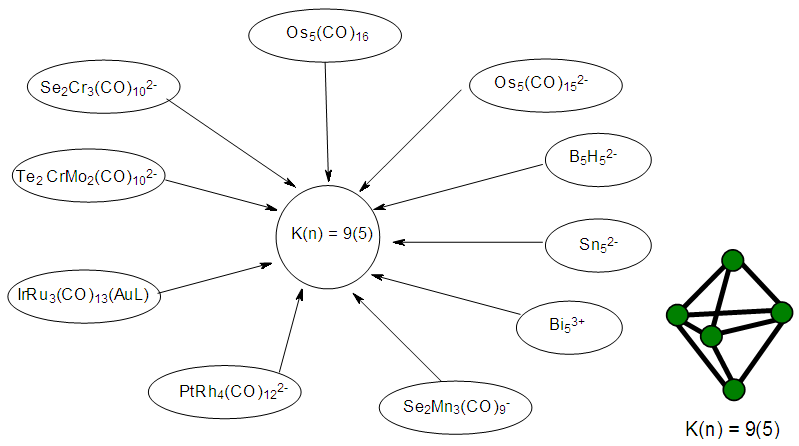 | Figure 3. Selected Clusters with Trigonal Bipyramid Shape |
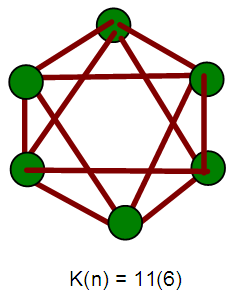 | Figure 4. An ideal skeletal octahedral Shape |
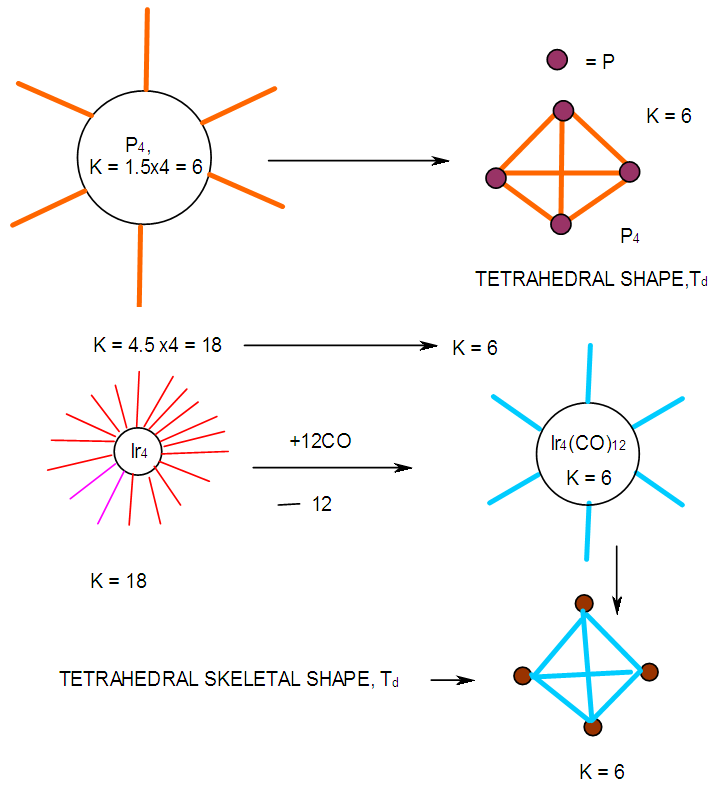 | Figure 5. Selected clusters with similar skeletal shape |
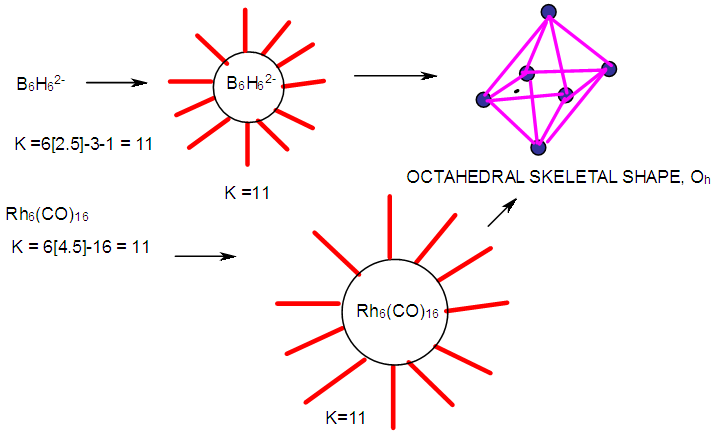 | Figure 6. Selected Clusters with similar skeletal shape |
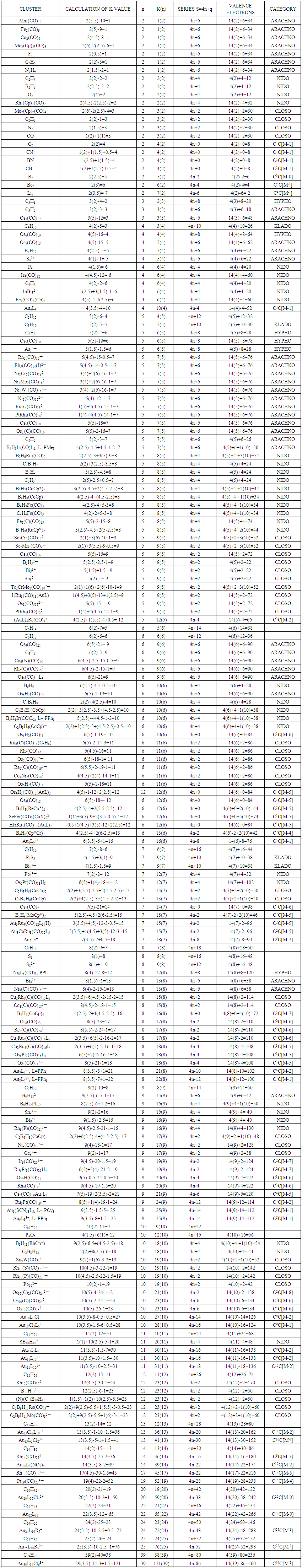 | Table 3. Categorization of Clusters using K(n) parameter |
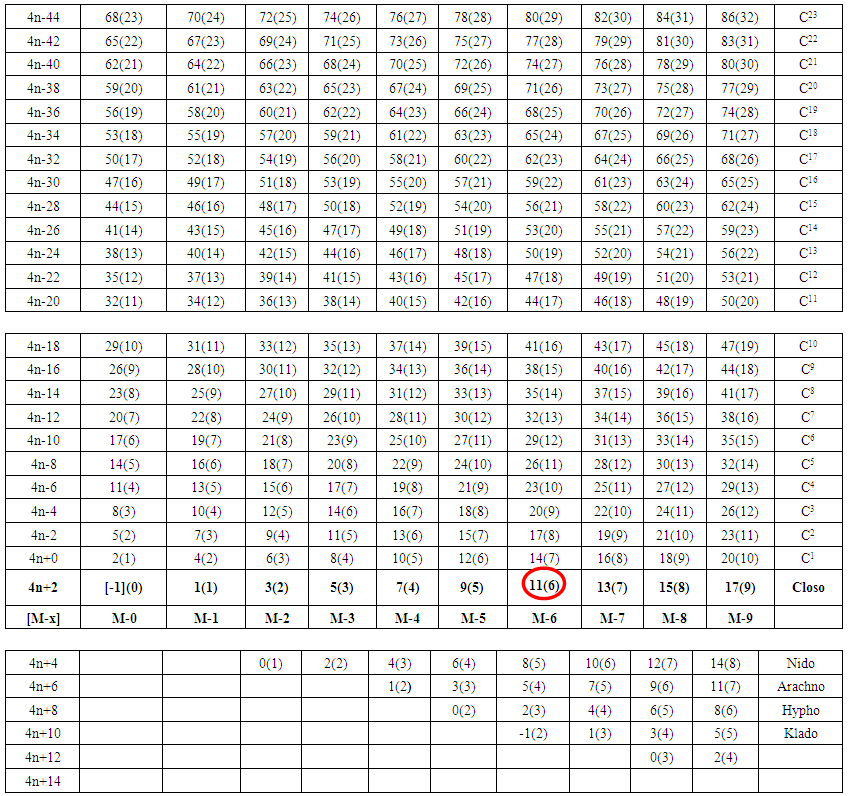 | Table 4. The K(n) Series for Cluster Categorization |
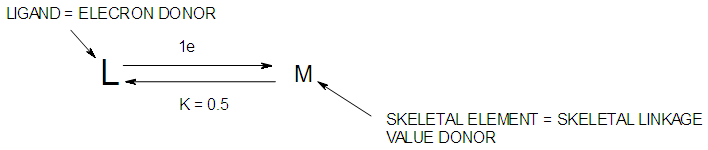 | Figure 7. Symbiotic relationship between a ligand and a skeletal element |
|
2.5. The Fifth Law. The Law of K(n) Correlation Series (Table 4)
2.5.1. The Fifth Law is the Basis of Table 4
- Taking K(n) = 11(6) as a starting point for illustration, the point 11(6) represents octahedral cluster symmetries. Moving upwards (VERTICAL MOVEMENT) from 11(6) we reach 14(7). That is, 11(6)→14(7). The point 14(7) represents a mono-capped octahedral shape, C1C[M-6]. Examples include [35], Os7(CO)21 and Re7(C)(CO)21H2-. Further movement upwards gives the next point 17(8). That is, 14(7)→17(8). This gives us a bi-capped octahedral cluster, C2C[M-6]. Examples include [35] and Re8(C)(CO)242-, and Os8(CO)222-. Let us consider HORIZONTAL MOVEMENT TO THE RIGHT from 11(6). This will give the point 13(7) and the next point will be 15(8). On the other hand moving to the LEFT we get 9(5). That is, 9(5)←11(6). The point 9(5) represents the ideal geometry of trigonal bipyramid clusters. Both 9(5) and 11(6) belong to 4n+2 series (CLOSO). Let us consider the DIAGONAL MOVEMENT going to the left. From 11(6) we reach 12(6). That is, 11(6)→12(6). The next point is 13(6). The process gives rise to continuous capping series of a cluster without varying the number of cluster elements. Moving from 11(6) DIAGONALLY to the right gives the point 10(6) and the next point is 9(6). Whereas 11(6) code normally represents an OCTAHEDRAL GEOMETRY, the point 9(6) normally represents the ideal skeletal shape of TRIGONAL PRISM CLUSTERS (see Figure 7). Benzene, C6H6[K(n) =9(6) and prismanes C6R6[(R = H, alkyls or aryls, K(n) = 9(6)] also portray trigonal bipyramid prism geometry [37]. Other examples include [37], Rh6(C)(CO)152- and Rh6(N)(CO)15-. The three important types of correlations are sketched in Figure 8. These three types of cluster series are discussed in more details below.
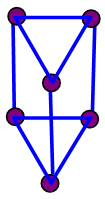 | Figure 7. Ideal skeletal shape of a trigonal bipyramid prism geometry |
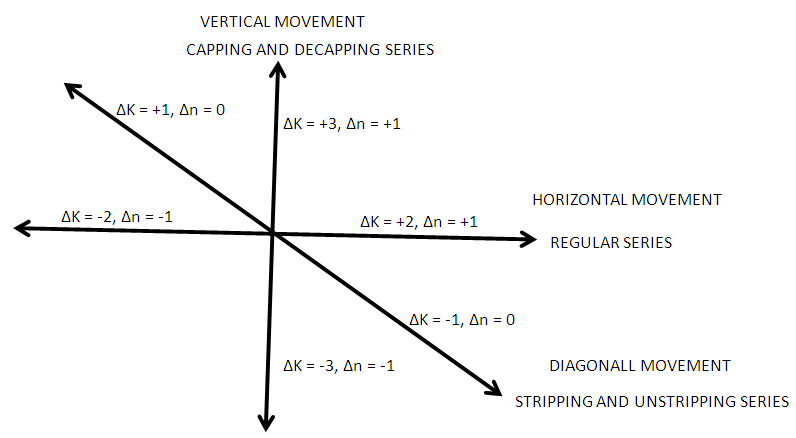 | Figure 8a. Three Major Types of K(n) series |
 | Figure 8b. Continuation |
2.5.2. The Vertical Movement in Figure8, Table 4, ∆K = 3, and ∆n = 1, Capping Series (Rudolph Series)
- Let us use the code K(n) = 11(6) as a reference point in Figure 8. The code sequence as we move upwards from the center will be as follows, 11(6)→14(7)→17(8)→20(9)→23(10)→26(11)→ 29(12)→ 32(13) and so on. These represent the capping series for an octahedral geometry. From 14(7), that is a code for a mono-capped octahedral cluster with a symbol, C1C[M-6]. Examples include [38] Os7(CO)21 and Re7(C)(CO)213-. The next, 17(8) represent a bi-capped octahedral cluster, C2C[M-6]and examples include [35] Re8(C)(CO)242- and Os8(CO)222-. With the third one, C3C[M-6], a tri-capped octahedral cluster, examples are [35] Os9(CO)242- and Rh9(CO)193-, and the code C4C[M-6] represents a tetra-capped octahedral cluster. The examples include [38] Os10(CO)262- and Rh10(CO)212-, and so on. If we go vertically down below K(n) = 11(6), we get K(n) = 8(5). Examples were given under the Fourth Law above. We can regard this code to represent a square pyramid geometry. If we carefully examine the vertical movements along the code numbers, we see clearly that we are actually reproducing and extending the vital concept observed by Rudolph [3] in his scheme widely utilized to categorize mostly borane clusters and their relatives.
2.5.3. The Horizontal Movement in Figure 8, ∆K = 2 and ∆n = 1, Ordinary Series
- Let us use the same code K(n) = 11(6) as our reference point for the illustration of horizontal movement according to Figure 8 and Table 4. Moving to the right from 11(6), we get the following sequences: 11(6)→13(7)→15(8)→17(9)→19(10), etc as mentioned above. On moving to the left we get: 11(6)→9(5)→7(4)→5(3)→3(3)→1(2). All these K(n) values belong to the same series S = 4n+2(CLOSO family). The code K(n) = 9(5) represents an ideal skeletal shape of a trigonal bipyramid as indicated in Figure 3. Examples were also mentioned under the Fourth Law. The code 3(3) usually represents an ideal triangular shape (∆) and 1(2) represents the ideal shape of two skeletal elements linked by one bond is shown in Figure 2[(i)].
2.5.4. The Diagonal Movement in Figure 8, ∆K = 1 and ∆n = 0, Stripping Series
- Let us consider a series of K(n) values where n is kept constant. Moving along the diagonal from the right beginning with 0(1), the next is 1(1) followed by 2(1), 3(1), 4(1) and 5(1); this could represent the stripping series of Fe(CO)5 (Scheme 1). The K(n) variations with n constant can be presented as a diagonal movement in Table 4. The constant n values may be regarded as ‘HIGHWAYS’. Thus, we have tabulated the selected highway values, n = 1, 2, 3, 4, 5, up to 32 shown in Table 4, which can be extended as desired. Selected examples of clusters lying on highways from n= 1 to n= 5 are given in Table 6. Let us use the code 11(6) as a reference point once more. A lot of good work on stripping series has been done by several research groups [16-19]. Some of the complex systems studied include: H3Ru4(CO)12―, K(n) = 6(4)→HRu4―, K(n)=19(4); PtRu5(C)(CO)152―, K(n) =11(6) →PtRu5(C)(CO)82―, K(n) = 18(6); Os10(C)(CO)242―, K(n) = 23(10)→Os10(C)2―, K(n) = 47(10); Pd6Ru6(CO)242―, K(n) =29(12)→Pd6Ru62―, K(n)=53(12) and Os20(CO)402―, K(n)=59(20)→Os202―, K(n) =99(20). Taking the stripping series of K(n) = 6(4)→19(4){H3Ru4(CO)12―, K(n) = 6(4)→HRu4―, K(n)=19(4)} as an example, we get 6(4)[S=4n+4]→7(4) [S=4n+2] →8(4) [S=4n+0] →9(4) [S=4n-2] →10(4) [S=4n-4] →11(4) [S=4n-6] →12(4) [S=4n-8] →13(4) [S=4n-10] →14(4) [S=4n-12] →15(4) [S=4n-14] →16(4) [S=4n-16] →17(4) [S=4n-18] →18(4) [S=4n-20] →19(4) [S=4n-22. Of great importance is that, when the diagonal relationship is analyzed carefully, all the K values of clusters with the same skeletal number of elements (n) lie along the same diagonal. For example, Ni6(Cp)6, K(n) = 9(6), Rh6(CO)15(C)2-, K(n) = 9(6), Re6H8(CO)182-, K(n) = 10(6), Rh6(CO)16, 11(6), and Os6(CO)18, K(n) = 12(6). The order is 9(6)→10(6)→11(6) →12(6). That is, the diagonal ‘Highway’ is along n = 6. Take another example, K(n) = 4(5)[C5H12]→5(5)[C5H10] →6(5)[C5H8] →7(5)[Rh5(CO)15―]→8(5)[B2H6Ru3(CO)9, C2B3H7,B5H9] →9(5)[B5H52-, Sn52-,Os5(CO)16, Se2Cr3(CO)102-]→10(5) →11(5) →12(5)[(AuL)4Re(CO)4+]. All these clusters are found lying along a diagonal highway n = 5 in Table 4. More examples of clusters that lie along the same diagonal are given in Table 6.
2.6. Cluster Hole Formalism
- The stripping of CO ligands from the cluster Os10(C)(CO)242―, K(n) = 23(10){4n-6→C4C[M-6]} to Os10(C)2―, K(n) = 47(10){4n-54→C28C[M-18]} cluster involves the loss of 24 CO ligands. According to series, this means an increase of K values by 24 units. As expected, the new K value will be 23+27 = 47. Hence, the K(n) values will follow the increasing trend as follows: 23(10)→24(10)→25(10)→26(10)→27(10)→28(10)→ 29(10)→30(10)→31(10)→32(10)→ 33(10)→34(10)→ 35(10)→36(10)→ 37(10)→38(10)→39(10)→40(10)→ 41(10)→42(10)→43(10)→44(10)→45(10)→46(10) →47(10) Øcorresponds to S = 4n-54→Cp = C28C[M-18]. Since we know that the cluster has 10 skeletal elements, and the capping symbol states there are 28 capping elements, this could possibly be translated as 10 skeletal elements co-existing with ‘18 holes’. It is likely that the presence of such holes in a cluster promotes the flow of electrons and hence electrical conductivity.
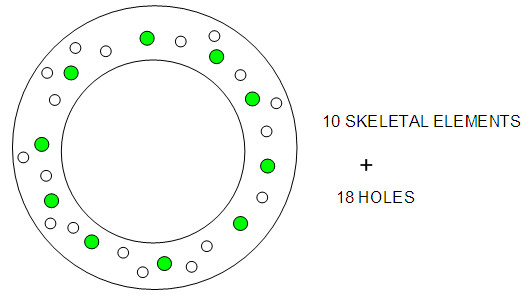 | Figure 9. Shows the possible presence of capping holes in the clusters |
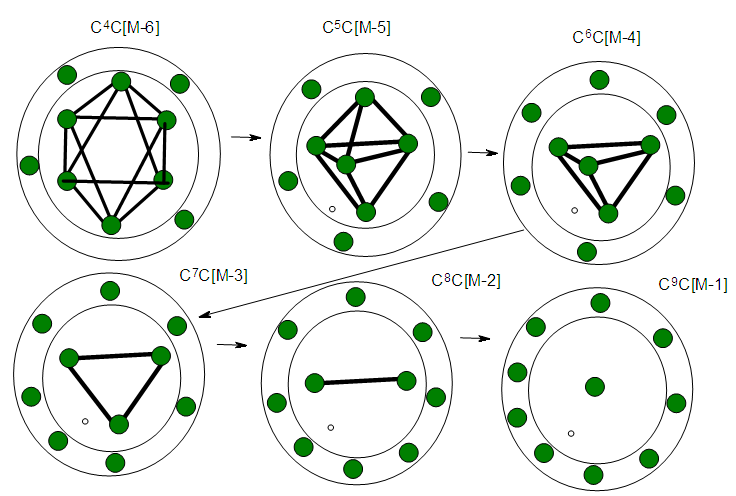 | Scheme 5. Shows increase in Capping with increased stripping of CO ligands |
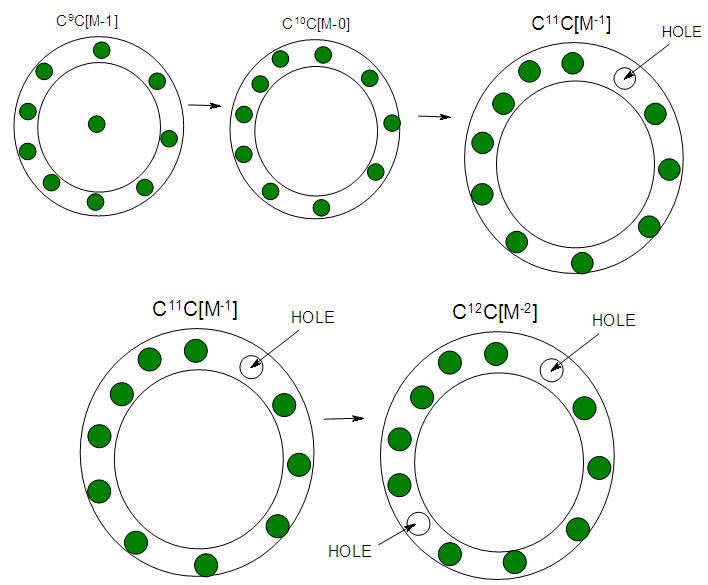 | Scheme 5. Continued |
|
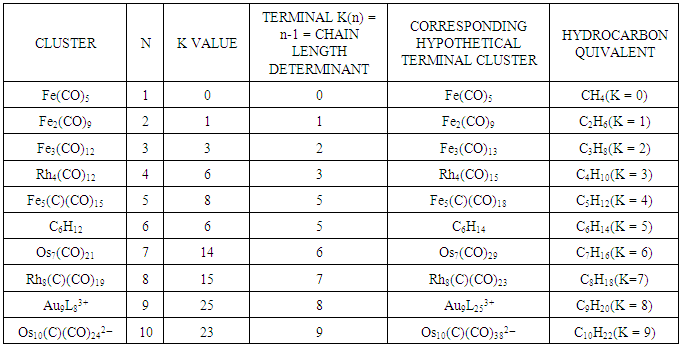
2.7. The Sixth Law: The Cluster Chain Length
- The chain length of a cluster Mn (n = number of skeletal elements) terminates when K = n-1. Scheme 6 gives a few examples to demonstrate the concept of cluster termination (saturation) point.
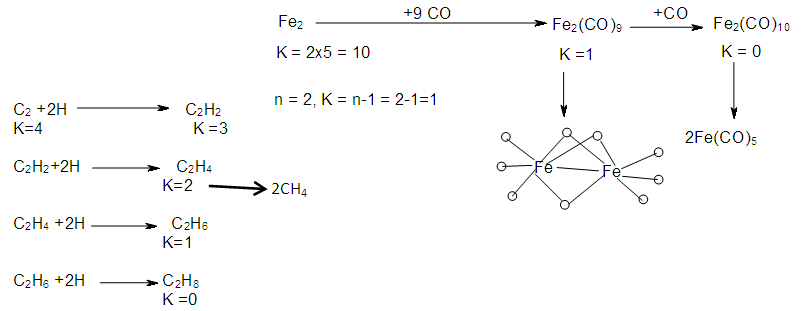 | Scheme 6. Creation of Cluster Chain Length |
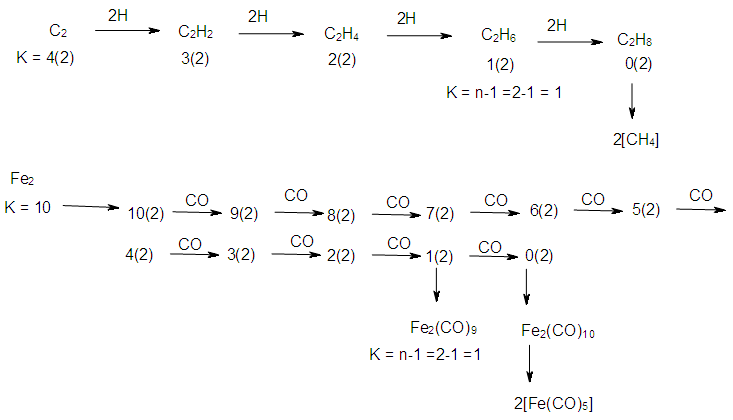 | Scheme 7. Stepwise decrease of K values with addition of ligands for a hydrocarbon and metal carbonyl cluster |
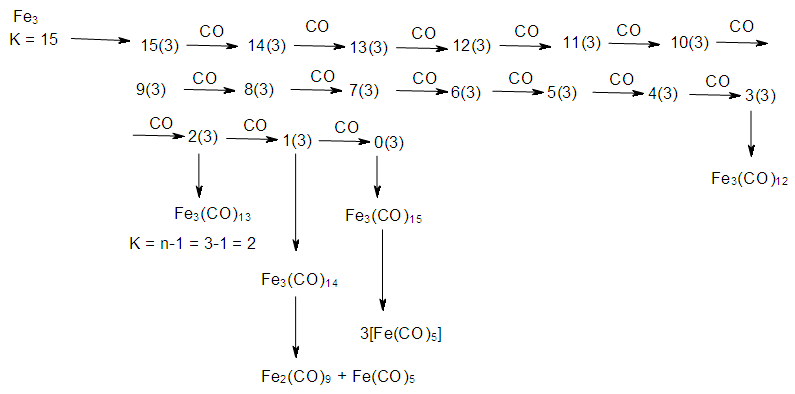 | Scheme 8. Possible Mechanism for cluster formation of Fe3 cluster chain length |
|
2.8. Categorization of Clusters Using Table 4
- Categorization of clusters using skeletal numbers and series has been discussed before [28-31]. However, in this paper categorization using cluster number (K value) and Table 4 will be applied. In the examples given below, the Law of conservation of cluster linkages is applied. They show the ease and quick approach to the derivation of cluster K values and their application with Table 4 to categorize clusters.1. Co6(C)(CO)12(AuL)4K =6[4.5] +4[2.5]-2-12 = 23→23 (10)→ S =4n-6 (from Table 2), Cp = C4C[M-6]. This means the cluster belongs to the tetra-capped series [36]. It has an octahedral nucleus as sketched in Figure 10.
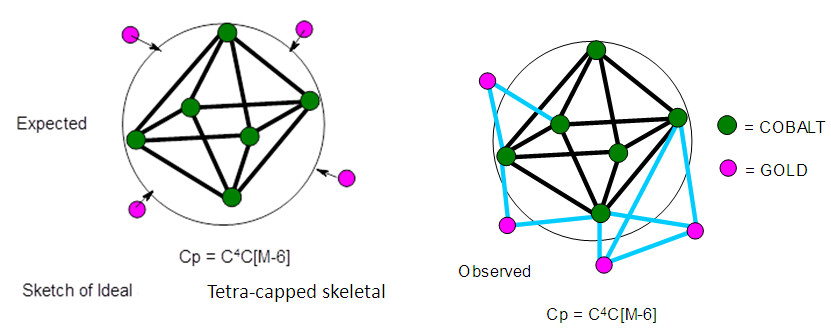 | Figure 10. Possible shape of a tetra-capped skeletal octahedron |
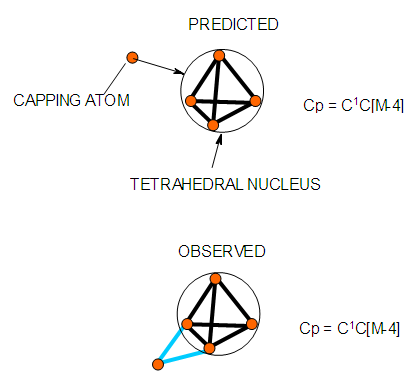 | Figure 11. Possible shape of a mono-capped tetrahedron |
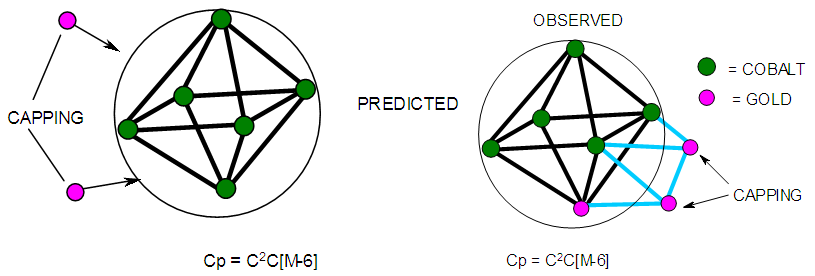 | Figure 12. Possible shape of a bi-capped octahedral shape |
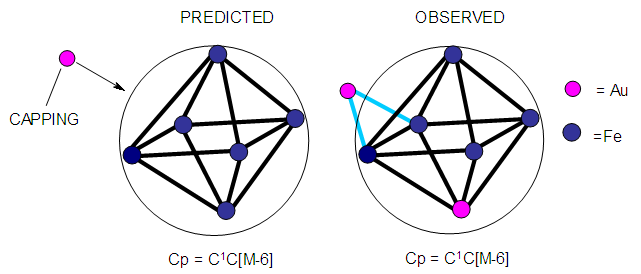 | Figure 13. Possible shape of a mono-capped octahedral shape |
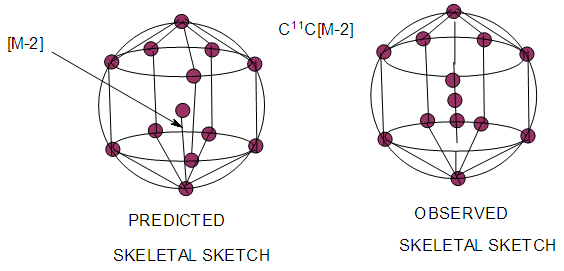 | Figure 14. A cluster with a nucleus containing two skeletal atoms |
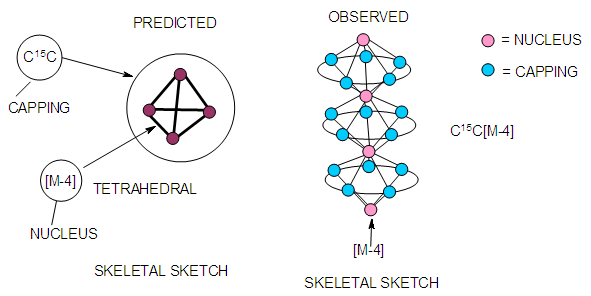 | Figure 15. A cluster with an ideal centered tetrahedral skeletal nucleus |
 | Figure 16. Sketch of an ideal trigonal bipyramid shape |
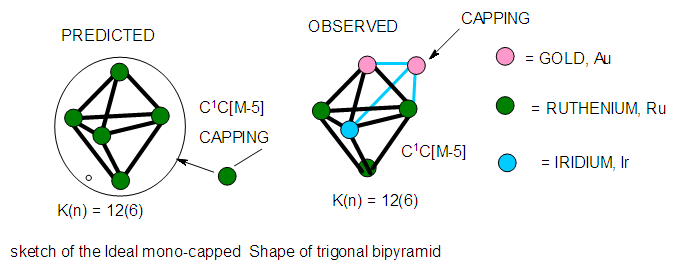 | Figure 17. Sketch of an ideal mono-capped trigonal bipyramid cluster |
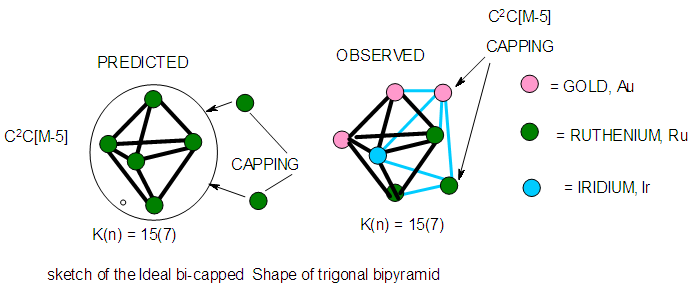 | Figure 18. Sketch of Ideal bi-capped trigonal bipyramid cluster |
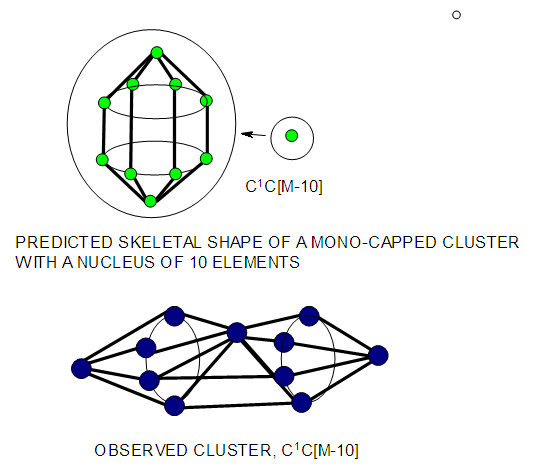 | Figure 19. Ideal mono-capped cluster with a 10 skeletal atom nucleus |
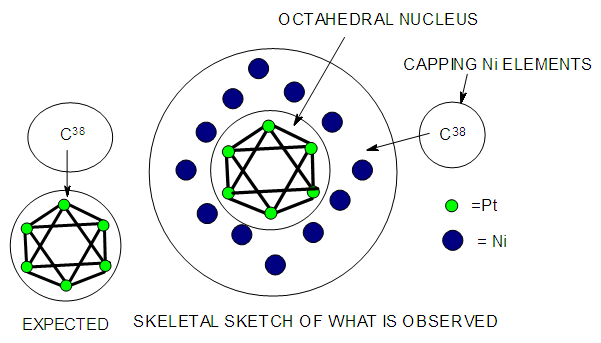 | Figure 20. Sketch showing a giant cluster with an octahedral nucleus |
3. Conclusions
- The six simple rules or laws of chemical clusters will make it much easier to analyze and categorize a wide range of clusters using skeletal linkage values and the K(n) parameter. Furthermore, the geometries of some clusters could readily be predicted. The skeletal cluster hole formalism concept has been introduced. The categorization of clusters using the K(n) parameter and the cluster mapping format in Table 4 has been demonstrated. The clear identification of the six rules or laws underpins the great importance of the use of the Series method, which could be applied to explain more complex clusters. These six chemical cluster laws are being unambiguously pronounced for the first time in the history of clusters.
ACKNOWLEDGEMENTS
- I wish to acknowledge the University of Namibia for providing the facilities and enabling environment to do this work and NAMSOV, NAMIBIA for financial support. Further, I wish to thank Dr. P.E. T. Kiremire and Mrs M.K. Kiremire for proof-reading the draft.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML