-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2017; 7(1): 16-20
doi:10.5923/j.chemistry.20170701.03

Comparative Study of the Copper Electrical Earthing Corrosion in Three Mediums in Senegal
E. H. A. Diop1, M. F. Niang1, D. Gassama2, N. P. Dione3, M. Fall4
1Doctoral School for Sustainable Development and Society, University of Thies, Senegal
2Training and Research Unit, Sciences and Technology, University of Thies, Senegal
3Groupe des laboratoires d’analyses (GLA), Direction des mines et de la promotion minière, IST 2° étage, Faculty of Sciences and Techniques, Cheikh Anta Diop University of Dakar, Senegal
4Department of Chemistry, Faculty of Sciences and Techniques, Cheikh Anta DIOP University of Dakar, Senegal
Correspondence to: E. H. A. Diop, Doctoral School for Sustainable Development and Society, University of Thies, Senegal.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

A electrical earthing is a low-resistance metallic electrode in close contact with the earth, which is able to conduct default and leaking electric current to the earth. A good electrical earthing allows current circulation trought the earth and protects equipements and persons from electrical risks. In Senegal a foundation earthing with a loop system onto the bottom of building excavation is used on a high majority of new buildings in construction. The same copper is use in all sites for all kinds of buildings (office, school, commercial or medical use). The corrosion resistance of the copper grounding in the soil is very important for the safety and power operation. A quick consumption of copper by corrosion with the presence of electrolyte (natural water exposure) can occurs a matter of safety. We purpose to study the corrosion rates in different water exposures. This work is about a comparative corrosion rates of a copper electrical earthing in diffferent mediums. Those mediums of study are the exposure of three aqueous environments: city water, rainwater and seawater. The purpose of the work is to assess and compare this earthing corrosion rates in those various environments and found the more aggressiveness solution or medium. The physico-chemical datas of aqueous solutions were analyzed and summurised before electrochemicals measurements. The open circuit potential (OCP) is accsess by chronopotentiometry after 2 hours of potential monitoring. The linear polarization with Evans digramm and Tafel extrapolation methods were performed. Those methods show the kinetics of corrosion of copper earthing in city water, seawater and rainwater. The results showed a higher corrosion rate in seawater (32 µm/year) and low corrosion rates in city water and rainwater (ranging about 2 and 3 µm/year). For more security of persons and equipments copper earthing require in sea areas more thickness or more control or replacements. This study contributes to increase the safety of electrical power in buildings.
Keywords: Copper electrical earthing, Rainwater, Seawater, City water, Corrosion rate
Cite this paper: E. H. A. Diop, M. F. Niang, D. Gassama, N. P. Dione, M. Fall, Comparative Study of the Copper Electrical Earthing Corrosion in Three Mediums in Senegal, American Journal of Chemistry, Vol. 7 No. 1, 2017, pp. 16-20. doi: 10.5923/j.chemistry.20170701.03.
Article Outline
1. Introduction
- A electrical earthing is a low-resistance metallic electrode in close contact with the earth, which is able to conduct default and leaking electric current to the earth with low frequency (low voltage network in 50 Hz alternating current) or high frequency (lightning current) as defined by Lim et al [1]. Those electrical earthing are used with a foundation earth electrode or a simple vertical earth rod in general. In the context of our study (Senegal), most of the earthing are in copper looping. Many works devoted to the study of those groundings and related to the soils’ resistivity reduction, thus permitting to improve the earthing quality (lower electric resistance) are available in the literature [2-11]. In contrast, only few works are focused between the correlation of the earthing corrosion and the increase of the earth’s [12] conductivity. In Senegal copper looping is genreraly use in all sites for new buildings for office, schools, hospitals, administration, banks, etc. Due to the importance of the corrosion resistance for durability and safety for persons and equipements it is necessary to show the corrosion rates of copper earthing for different mediums. A higher corrosion rate can contribute to a rapid consumption or a disappearance of the electrical earthing protection with negation consequence for humain and equipement safety. This paper sets out the corrosion rates in rainwater, city water and seawater of a 29 mm2 copper earthing. Due to the presence of water in soil our study show the influence of water quality (city water, rainwater and seawater) on the copper electrical earthing corrosion. The study can clarify if is judicious to use copper as a electrical earthing in all sites exposures. The criteria of corrosion rates comparison is use for solving our problematical. Three methods were used for investiguations. First the physico-chemicals analysis allow to show different parameters of aqueous solutions as temperature, ph, conductivity, total hardness, anions and cations concentrations. The second method is the chronopotentiometry in zero current for determination of the free-corrosion potential or open circuit potential (OCP) of copper in the mediums of study for 2h. The third method set out the Evans and Tafel extrapolations diagrams with the corrosion by polarization for ± 500 mV Ag/AgCl around the OPC. Experimental results will permit to compare the copper corrosion rates in the different environments and set out the most aggressiveness medium.The study can be benefict for electrical technicians and humains to increse the safety in many buildings in Senegal.
2. Experimental
2.1. Material
- The electrochemical measurements were performed on 29 mm2 samples cut from pure (99%) bare copper of earthing. These copper samples were used as working electrodes, Ag/AgCl in saturated KCl was used as the reference electrode, and the counter-electrode was a large stainless steel grid.
2.2. Solutions
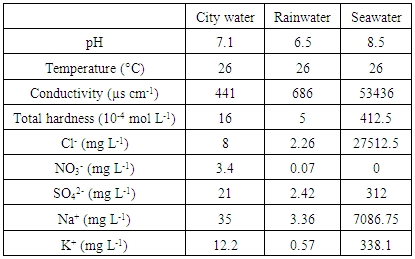
- Basically, buried metal corrosion, has an electrochemical nature due to the presence of electrolyte in the earth [13]. The solutions under study are city water, seawater and rainwater. The physicochemical characteristics of these solutions are summarized in Table 1. The ph, temprerature and conductivity were determined by measurements with standarts equipement. The others parameters were carry out by calorimetry.We should mention that rainwater used in this study was directly collected, before any contact with the land. This rainwater is different from the other run-off or infiltration waters definitions [14]. Those waters can be loaded with various contaminants like hydrocarbons from paved roads deteriorations, minerals, organic components or microorganisms, etc. [15, 16].
|
2.3. Experimental Methods
- The copper sample corrosion was investigated by chronopotentiometry and linear dynamic polarization methods [17] using a Potentiostat/Galvanostat µ-Autolab III controlled by the GPES (General Purpose for Electrochemical System) software. The external surfaces of the metal samples were first sandpapered with abrasive papers (P 300 and P 800 successively) to remove the surface oxides layers before rinsing them using a squeeze bottle of distilled water. The open-circuit potential was determined by chronopotentiometry for 2 hours, after a period of stabilization of 30 minutes. Linear polarization (Evans diagram and Tafel extrapolations) consists of polarizing the electrode in the domain Ecorr ± 500 mV, then in fitting the experimental values into the Tafel lines. This method permits to obtain the corrosion kinetics datas (corrosion rate, potential and current of corrosion, Tafel slopes, etc.) [18]. The measurement is done subsequently to the free-corrosion potential measurement. The scanning rate was 1 mV s-1 in the explored potential field for 1 cycle by linear polarization for ± 500 mV Ag/AgCl around the OCP. The samples surfaces were measured and specified in the operating software datas.
3. Results and Discussion
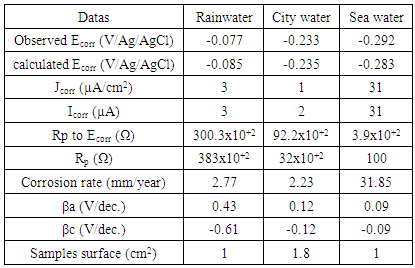
- Free-corrosion potential monitoring in various environments, as shown in Fig. 1., is more anodic in rainwater with a value close to -0.05 V/Ag/AgCl. In this case, the potential slightly increases from -0.06 to -0.04 V/Ag/AgCl between 0 and 1470 seconds. This step may correspond to a passivation of the metal due to the formation of a passive film on the electrode. From 1470 to 7200 s, the potential is almost constant (≈ -0.045 V/Ag/AgCl). The low presence of aggressive halogen anions and the low conductivity in rainwater (Table 1) are compatible with a low tendency for corrosion which explains the more anodic potential in this solution. Fig. 1 shows that the most cathodic free-corrosion potential is obtained in seawater (about -0.22 V/Ag/AgCl). The potential rapidly decreases (from -0.12 to -0.22 V/Ag/AgCl) from 0 to 540 seconds in the first minutes, may be due to the dissolution of an oxide film that was formed on the surface [19]. From the 540 seconds to 7200 s, the potential stabilizes at around -0.22 V Ag/AgCl. The copper turns passive in this solution in which a blue coloration appears indicating a formation of Cu2+ ions. The important chloride concentration in seawater and its high conductivity (Table 1) explain the seawater aggressiveness (corrosion) [1] and the more cathodic potential. Fig. 1 shows that the free-corrosion potential in city water (about -0.18 V/Ag/AgCl) is between those in seawater and rainwater. From 0 to 368 seconds, the potential decreases from -0.12 to -0.19 V/Ag/AgCl, and remains quite constant after (-0.18 V/Ag/AgCl). As for the ions concentrations, the potential value is comprised between those obtained in seawater and rainwater, Anions in city water are related to human consumption water's treatment products. The corrosion kinetics was also investigated by plotting Evans Diagrams and Tafel extrapolations in the various environments.Fig. 2 confirms that the most cathodic corrosion potential is obtained in seawater (about -0.29 V/Ag/AgCl) and the most anodic one in rainwater (about -0.08 V Ag/AgCl). The corrosion potential in city water is between the values obtained in rainwater and seawater (about -0.23 V Ag/AgCl). This is in accordance with the free-corrosion potential measurement results. The anodic branch shows a more important corrosion current density in seawater with an increase of 2.78 µA/cm2 to 0.018 A/cm2 between -0.29 and 0.09 V Ag/AgCl potentials. The anodic branch is round shape in rainwater corresponding to an increase of current density from 0.04 µA/cm2 to 29.6 µA/cm2 between 0.07 to 0.38 V Ag/AgCl potentials. In city water, the anodic branch follows the same behaviour as into rainwater with a part which current density increase is more rapid (0.05 µA/cm2 to 110 µA/cm2) between -0.22 to 0.12 V Ag/AgCl potentials. The slopes of anodic curves (Fig. 2) are more important in city water and in rainwater. Then the courant of corrosion is more emphasized, respectively in seawater. The additions of material like salt or components rich in chloride improve the soil conductivity (as in seawater) and contribute to increase copper earthing corrosion [1]. This analysis is in line with the conclusions obtained on the free corrosion potentials study. The Tafel datas analysis in Table 2 show that the corrosion rate in seawater (31.85 µm/year) is about 14 times moreimportant than the one obtained in city water (2.23 µm/year) and 11 times higher than rainwater (2.77 µm/year).
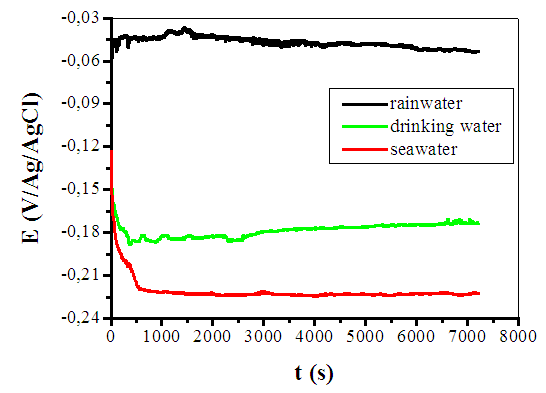 | Figure 1. Copper free-corrosion potential evolution for 2 hours in the three studied environments |
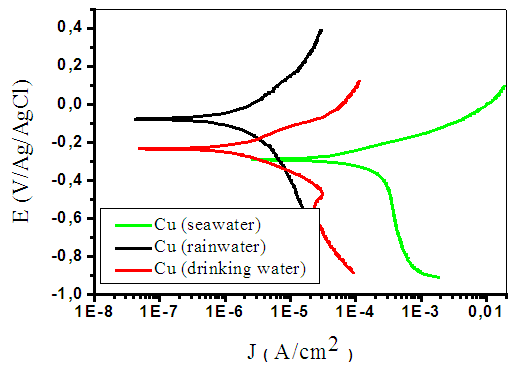 | Figure 2. Evans diagram of copper in various mediums |
|
4. Conclusions
- The study show a electrical risk for persons and equipements with higher corrosion of copper earthing in sea areas as in Senegal context. Copper potential is more cathodic into seawater (presence of chlorides), and more anodic in rainwater. The corrosion rate is especially higher in seawater (32µm/year). It is at least ten times more intense relatively to city water or rainwater. Copper earthing corrosion into city water and rainwater is quite the same according to the Tafel results (ranging about 2 and 3 µm/year). Then the use of copper as a general material of electrical earthing in all sites as in Senegal is not judicious. The extended use of copper as a electrical earthing raises the issue of durability and must go together with specific measures into seawater (more important thickness or more frequent checking). Thus, soil conductivity improvement solutions through addition of material like salt or components rich in chloride will contribute to increase copper earthing corrosion. This solution is not good for copper corrosion in spite of the reducing of the resistance of copper earthing. As in sea areas this method increasing the conductivity of soil is not judicious due to the risk of important corrosion.Ours study shows a risk of higher corrosion in sea area and in earthing with clhlorides addition. The risk is a disparution of earthing by corrosion and compromise the function of electrical earthing with copper corrosion. The results contribute to increase more safety of copper earthing in electrical power for humains and equipments in the buildings.The study’s opened up prospects can include: • The impact of bacteria [12] and other soils components influence (acidity, porosity, etc.) on the results. Bacteria that could worsen corrosion through their metabolism and acid secretions [1] or inhibit corrosion by the biofilm formed [21]; soils that can be rich in limestone or phosphate able to suppress corrosion (inhibition) [1].• Increasing the number of samples (from 5 to 10) of study for more analytical datas. Corrosion in well water and waste water can be investigated.• A comparative study of corrosion in the sames mediums with stainless steel. Thus can inform to the relevance of the use of 304 stainless steel in earthing lightning protection as recommended by French standard recommendation [22], in aggressive soils.
ACKNOWLEDGEMENTS
- The authors would like to thank Mrs DIENE, Groupe des laboratoires d’analyses (GLA), Direction des mines et de lapromotion minière, former BRGM laboratory for carrying out the aqueous solutions chemical analysis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML