-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2017; 7(1): 6-15
doi:10.5923/j.chemistry.20170701.02

Inhibition of the Acid Corrosion of Zinc by Cannabis Plant Extract
S. El-Housseiny
Chemistry Department, Faculty of Science, Alexandria University, Egypt
Correspondence to: S. El-Housseiny, Chemistry Department, Faculty of Science, Alexandria University, Egypt.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The effect of extract of cannabis plant on the corrosion of zinc in aqueous 0.5M sulphuric acid was investigated by electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization techniques. EIS measurements showed that the dissolution process of zinc occurs under activation control. Potentiodynamic polarization curves indicated that the plant extract behaves as mixed-type inhibitor. The corrosion rates of zinc and the inhibition efficiencies of the extract were calculated. The results obtained show that the extract solution of the plant could serve as an effective inhibitor for the corrosion of zinc in sulphuric acid media. Inhibition was found to increase with increasing concentration of the plant extract. Theoretical fitting of different isotherms, Langmuir, Flory–Huggins, and the kinetic–thermodynamic model were tested to clarify the nature of adsorption. Effect of temperature on the inhibitive action of cannabis extract for the corrosion of zinc in 0.5M sulphuric acid was investigated and the activation parameters of the corrosion process in absence and presence of cannabis extract were calculated.
Keywords: Zinc, EIS, Inhibitor, Polarization, Acidic corrosion
Cite this paper: S. El-Housseiny, Inhibition of the Acid Corrosion of Zinc by Cannabis Plant Extract, American Journal of Chemistry, Vol. 7 No. 1, 2017, pp. 6-15. doi: 10.5923/j.chemistry.20170701.02.
Article Outline
1. Introduction
- Zinc is known to play a major role in corrosion protection of steel structures. To enhance the corrosion resistance of the zinc layer applied for steel protection [1, 2], conversion films are commonly produced on its surface. Although chromate has been successfully applied for many years, the fact that it is toxic and potentially carcinogenic has led to the development of alternative non-toxic corrosion inhibitors, such as silicate, molybdate, rare earth metal salts and some organic compounds [3-14]. Consequently, attention has been focused on the corrosion inhibiting properties [15-33] of natural products from plants. As natural products, they are a source of non-toxic, eco-friendly, readily available and renewable inhibitors for preventing metal corrosion. Several investigations have been reported using such economic plant extract. Earlier, Barannik and Putivlova [34] showed that the actual inhibitors in the plant extracts are usually alkaloids and other organic nitrogen bases, as well as carbohydrates, proteins and their acid hydrolysis products. The existing data show that most organic inhibitors act by adsorption at the metal/solution interface. The adsorption process depends on the electronic characteristics of the inhibitor, the nature of the surface, the temperature and pressure of reaction, steric effect, multilayer adsorption and a varying degree of surface site activity. The aim of present work is to test extract of cannabis plant as inhibitor for the acidic corrosion of zinc and discuss their inhibition mechanism.
2. Experimental
- Electrochemical impedance and polarization measurements were achieved using frequency response analyzer (FRA)/potentiostat supplied from prostate instrument. The frequency range for EIS measurements was 0.1 × 104 Hz with applied potential signal amplitude of 10 mV around the rest potential. The data were obtained in a three- electrode mode; platinum sheet and saturated calomel electrodes (SCE) were used as counter and reference electrode. The material used for constructing the working electrode was zinc that had the following chemical composition (% wt) 0.00001% As, 0.001% S, 0.001% P, 0.001% Pb, 0.005% Fe, 99.98% Zn was used for the electrochemical corrosion studies in aqueous solutions. The working electrode was fabricated by cutting and shaping them in cylindrical forms. A long screw fastened to one end of the test cylinder for electrical connection. The Teflon gasket thereby forms a water-tight seal with the specimen electrode that prevents ingress of any electrolyte and thus avoiding crevice effect. The leak-proof assembly exposes only glass, only one side of rod was left uncovered as constant surface area in contact with the solution. The sample was wet hand-polished using different grade emery papers 320, 400, 600, and 800 grit finishes starting with a coarse one and proceeding in steps to the fine grit up to a mirror finish, washed thoroughly with double-distilled water and finally dried by absolute ethanol, just before immersion in the solution. Each experiment was carried out with newly polished electrode. Before polarization and EIS measurements, the working electrode was introduced into the test of solution and left for 10 min to attain the open circuit potential (ocp) at which the change of ocp with time is 2 mV/min, i.e., the system had been stabilized. The polarization curve measurements were obtained at scan rate of 20mV/min starting from cathodic potential (Ecorr -250 mV) going to anodic direction. All the measurements were done at 30.0 ± 0.1°C in solutions open to the atmosphere under unstirred conditions. To test the reliability and reproducibility of the measurements, duplicate experiments were performed in each case of the same conditions.Optical micrographs will be taken by using Euromex optical microscope, with colour video camera that is connected to a personal computer.Solution preparationThe test solutions were prepared from analytical grade reagents and distilled water: 98% H2SO4 was purchased from Aldrich chemicals. Stock solution of plant extracts was obtained by the flowering tops of plants. A 100 g of dry cannabis plant (the flowering tops of plants), that was obtained by permission from public prosecutor, was minced into very small pieces. The minced plant was boiled with water for 5 minutes to remove chlorophyll and water soluble compounds. The solution was filtered and boiled water was discarded. The process was repeated several times until the final discarded boiled water becomes clear. The minced plant was left to dry in fresh air at room temperature. The dry minced plant was refluxed with 100 ml of petroleum ether. The minced plant solution was then filtered through number 1 Watman filter paper. The extract was evaporated to obtain cannabis residue [35]. This residue was dissolved in 100ml ethanol giving the stock solution of cannabis plant. The concentration of the stock solution was determined by evaporating 10ml and weight the residue [36]. The concentration of the stock solution was expressed in terms of gram per liter. For corrosion measurement in aqueous 0.5 M sulphuric acid solution, the cannabis residue was used to prepare different concentrations of cannabis extract in presence of containing 20% ethyl alcohol.
3. Results and Discussions
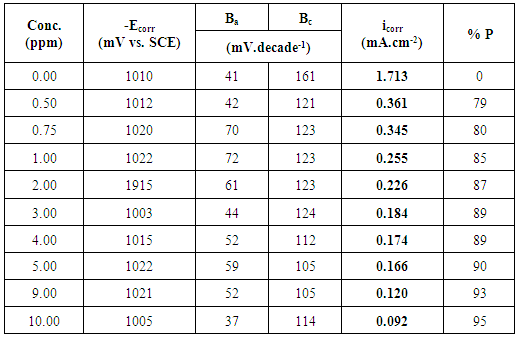
3.1. Potentiodynamic Polarization Results
- Figure 1 shows the potentiodynamic polarization curves of zinc in 0.5M sulphuric acid, in absence and presence of different concentrations of cannabis extract. As seen from this figure addition of the cannabis extract affects both the cathodic and anodic polarization curves indicating that the cannabis extract could be classified as mixed-type inhibitor. In general, for active metals such as zinc in acid solutions, when dissolved oxygen is present, both hydrogen evolution and oxygen reduction reactions will be possible. However, in view of the fact that, the saturated solubility of oxygen in pure water at 25°C is only about 10-3 mol dm-3 [37] and decreases slightly with concentration of dissolved salts. In addition, the concentration of H3O+ in acid solutions, at pH ≈ 0, is high, and since this ion has a high rate of diffusion, consequently, the contribution of the hydrogen evolution reaction at the cathodic process will overcome the oxygen reduction reaction. Therefore, the corrosion of zinc in acid solution proceeds via two partial reactions [38]:
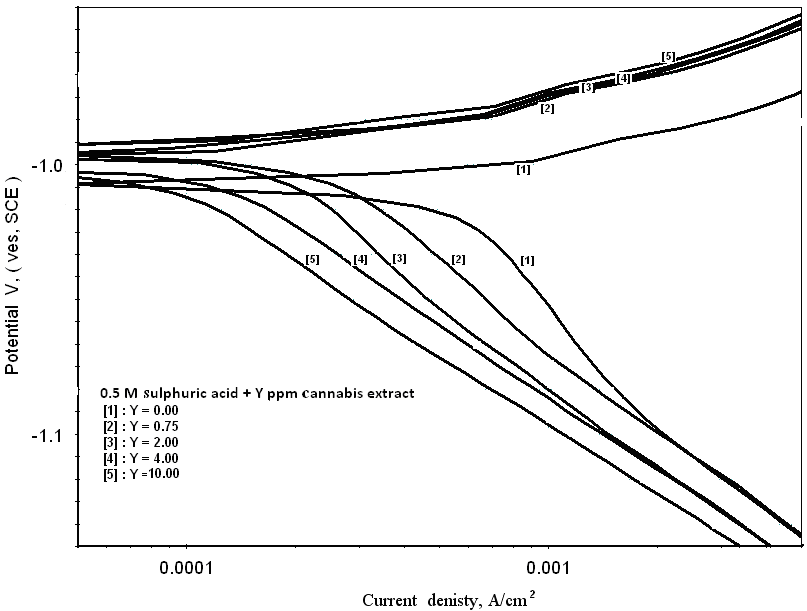 | Figure 1. Potentiodynamic polarization curves of zinc in 0.5M sulphuric acid, in absence and presence of different cannabis extract concentrations |
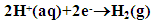 | (1) |
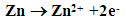 | (2) |
|
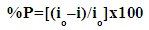 | (3) |
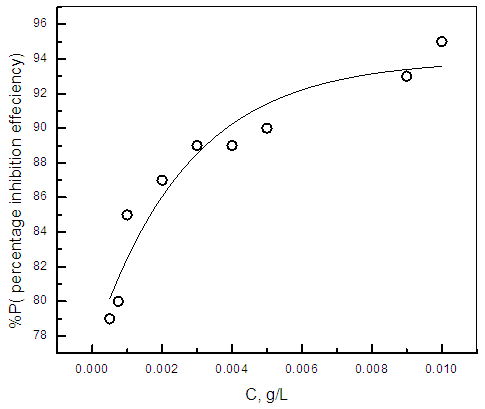 | Figure 2. Relation between the percentage inhibition efficiency and concentration of cannabis extract for zinc in 0.5M sulphuric acid solution |
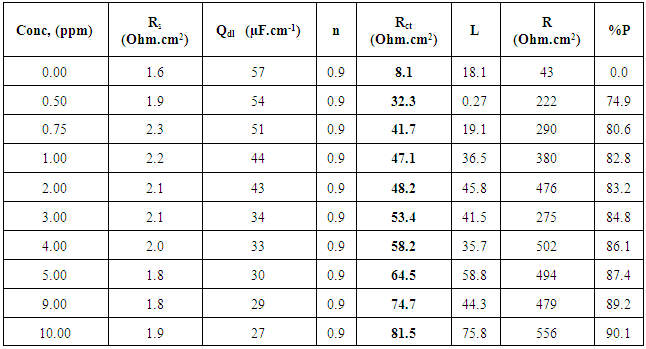
3.2. Electrochemical Impedance Spectroscopy (EIS) Results
- Figure 3 shows the Nyquist impedance plots of zinc in 0.5M sulphuric acid, in absence and presence of different concentrations of cannabis extract. The Nyquist impedance plots of zinc metal shown in this figure explain that the impedance response consists of capacitive semicircle followed by inductive loop indicating that the dissolution process occurs under activation control. The inductive loop is generally attributed to the adsorption of the species resulting from metal dissolution and hydrogen [42]. As seen, the size of the semicircle, increases with increasing cannabis extract concentration.
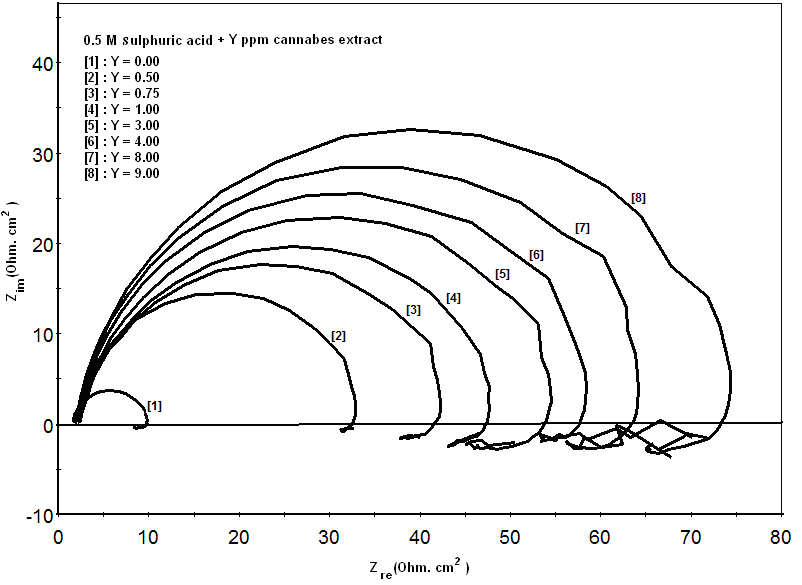 | Figure 3. Nyquist plots of zinc in 0.5M sulphuric acid, in absence and presence of different concentrations of cannabis extract |
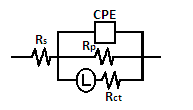 | Figure 4. Schematic for the equivalent circuit of zinc |
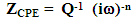 | (4) |
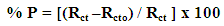 | (5) |
|
3.3. Optical Micrograph Results
- The optical micrographic photos of the zinc samples were captured using optical microscope with magnification power of 40X. Figure 5 shows optical micrographic photos of zinc in 0.5M sulphuric acid free from or containing cannabis extract after 6 hours immersion. As seen from the micrographic photos, the scratch mark of the emery paper still viewed within the experimental exposure time for zinc that was immersed in the acid solution containing cannabis extract whereas in the medium free from the extract, the scratch marks of zinc disappeared due to severe uniform corrosion.
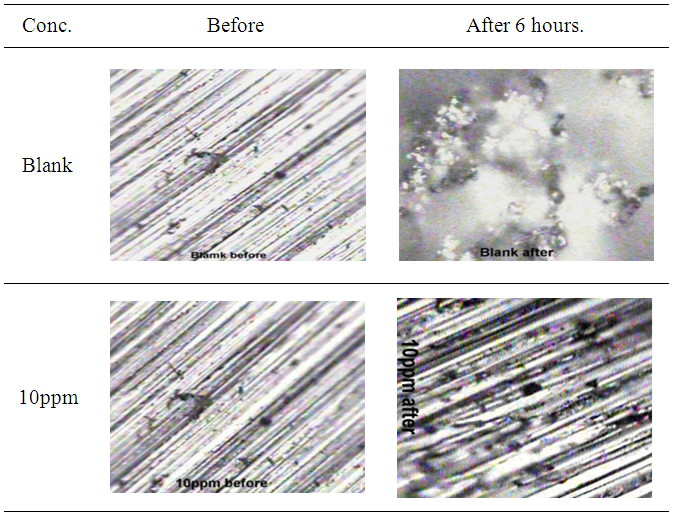 | Figure 5. Optical micrographic photos (40X) of zinc in 0.5M sulphuric acid, in absence and presence of cannabis extracts |
3.4. Application of Adsorption Isotherms
- The understanding of the nature of the adsorption process of various kinds of extracts on metal surfaces was essential to our knowledge of their inhibition action on corrosion. The action of an inhibitor in the presence of aggressive acid media is assumed to be due to its adsorption [45] at the metal/solution interface. The inhibition action was regarded as simple substitutional process [46, 47], in which an inhibitor molecule in the aqueous phase substitutes an x number of water molecules adsorbed on the metal surface, viz.
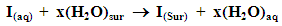 | (6) |
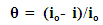 | (7) |
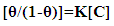 | (8) |
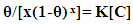 | (9) |
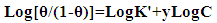 | (10) |
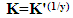 | (11) |
 | Figure 6. Linear fitting of the data of cannabis extract to Langmuir isotherm for zinc |
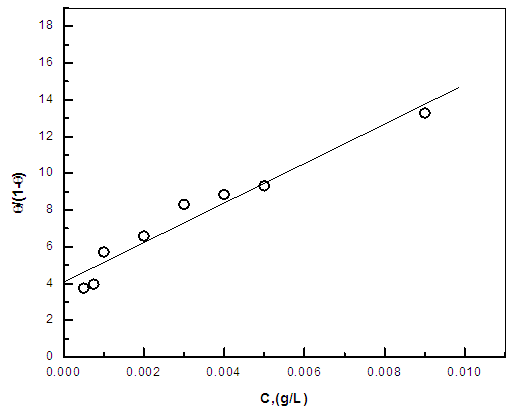
 | Figure 7. Linear fitting of the data of cannabis extract to Flory Huggins isotherm for zinc |
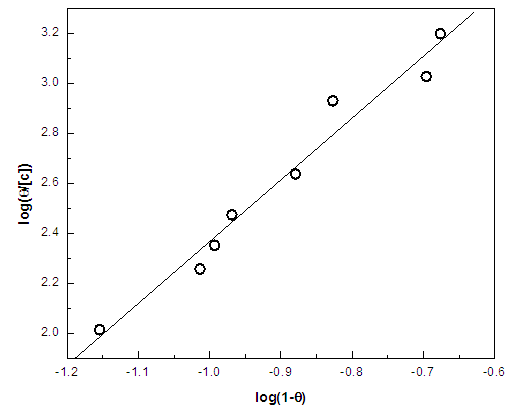
 | Figure 8. Linear fitting of the data of cannabis extract to Flory Huggins isotherm for zinc |
|
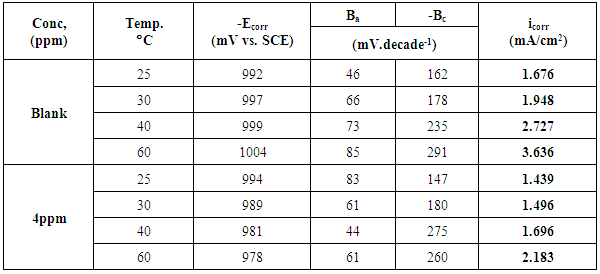
3.5. Effect of Temperature on the Inhibitive Action of Cannabis Extract for the Corrosion of zinc in 0.5M Sulphuric Acid
- Many industrial processes take place at high temperatures so, it is particularly important to study the variation of the inhibition efficiency with temperature. When temperature is raised, corrosive action is usually accelerated, particularly in media where evolution of hydrogen accompanies corrosion. Raising the temperature will decrease the inhibitor adsorption on the metal surface; consequently, it will lose its protective action. Cannabis was used in this investigation as an example of plant extract, as a corrosion inhibitor. Figures (9, 10) show the potentiodynamic polarization curves of zinc in 0.5M sulphuric acid, in absence and presence of cannabis extract at different temperatures. It is evident that increasing temperature affects anodic and cathodic parts of the polarization curves but by different degree depending on the solution composition. The values of the electrochemical parameters of zinc in 0.5M sulphuric acid, in absence and presence of cannabis extract at different temperatures are given in table 4. The data show that increasing temperature lead to increase of the corrosion current density (icorr).
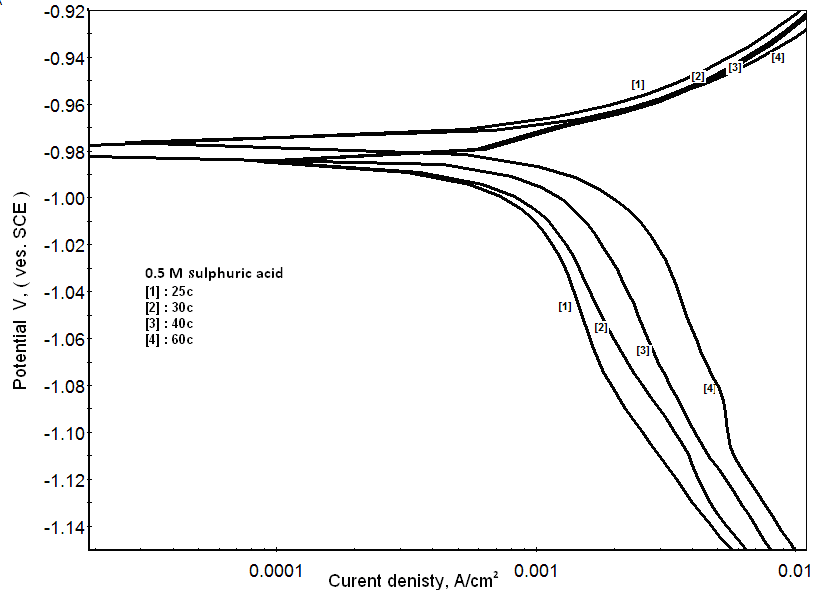 | Figure 9. Potentiodynamic polarization curves of zinc in 0.5M sulphuric acid, in at different temperatures |
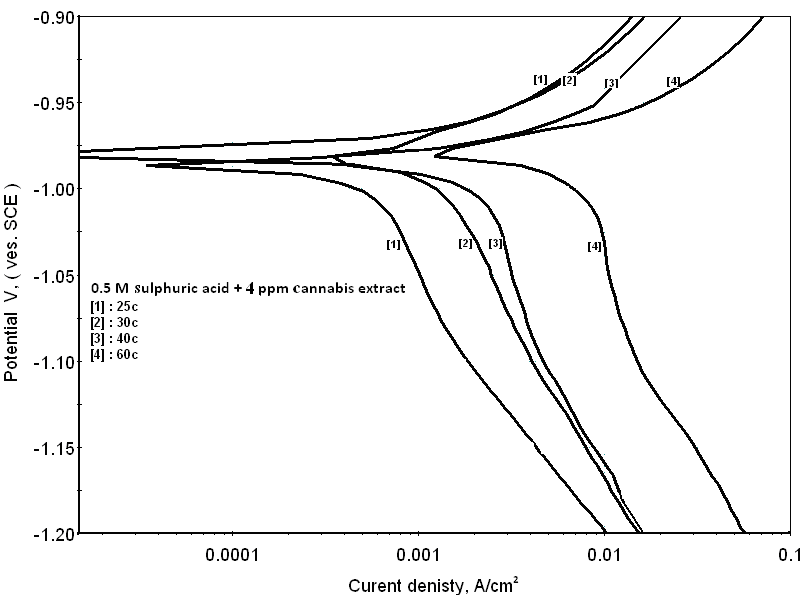 | Figure 10. Potentiodynamic polarization curves of zinc in 0.5M sulphuric acid, in presence of 4ppm of cannabis extract at different temperatures |
|
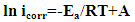 | (12) |
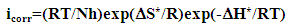 | (13) |
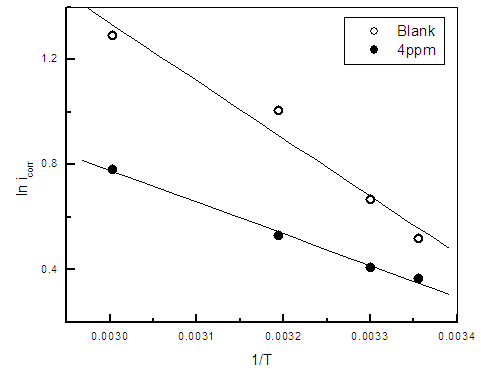 | Figure 11. Linear Square fit for zinc of ln icorr vs. (1/T) |
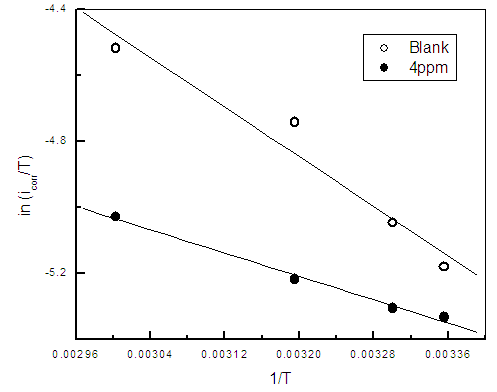 | Figure 12. Linear Square fit for zinc of ln (icorr/T) vs. (1/T) |
|
4. Conclusions
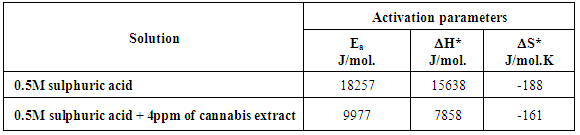
- 1. Cannabis extract act as efficient mixed type inhibitor for the corrosion of zinc in 0.5M sulphuric acid. 2. Inhibition was found to increase with increasing concentration of the cannabis extract. 3. EIS measurements showed that the dissolution process of zinc occurs under activation control. 4. Flory-Hyggins isotherm and Kinitic-Thermodynamic model were found to be applicable to fit the data of adsorption of cannabis at the zinc surface. The data showed that the adsorbed species of cannabis extract are bulky and two adsorbed water molecules were displaced by each species. 5. The values of Ea and ΔH* for dissolution of zinc in sulphuric acid solution containing cannabis extract are lower than in its absence indicates a chemisorption mechanism of adsorption.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML