-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2016; 6(6): 149-154
doi:10.5923/j.chemistry.20160606.01

Physico-Chemical Analysis of Wetland Water of Iba Community in Lagos State Nigeria
Bamgboye O. Agbeke1, Osundiya M. Olubunmi2, Olowu R. Adewale2, Adeniyi David A.2
1Department of Chemistry, Covenant University, Ota, Nigeria
2Department of Chemistry, Lagos State University, Lagos, Nigeria
Correspondence to: Olowu R. Adewale, Department of Chemistry, Lagos State University, Lagos, Nigeria.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The pollution level of wetland water in Iba area of Lagos state was studied. Water samples were collected from different spots on the wetland three times a week for a period of six weeks and analyzed for pH, total dissolved Solid (TDS), chemical oxygen demand (COD), alkalinity, acidity, chloride, nitrite, phosphate, sulphate, dissolve oxygen (DO), biochemical oxygen demand (BOD), calcium, sodium, iron, zinc, potassium, cobalt, nickel, cadmium, and chromium respectively. The trace metals were investigated using atomic absorption spectrophotometer (AAS). The average values of 6.30 ± 0.01 and 6.40 ± 0.01 were obtained for the pH of the wetland water during the normal and raining atmospheric conditions which is an indication that the wetland ecosystem is less acidic and the pH is within the set standard of 6.50-8.50 for portable water. The average values obtained for the physicochemical parameters ranged from 28.050- 28.433mg /L temperature, 6.36-6.40 mg /L pH, 57.05-56.97 mg /L total dissolved solid, 46.00-46.70 mg /L chemical oxygen demand, 84.42-84.67 mg /L alkalinity, 4.53-4.62 mg /L acidity, 130.53-130.95 mg /L, 5.10-5.12 mg /L chloride, 5.20-5.12 mg/L nitrite, 1.04-1.05 mg /L phosphate, 100.60-100.83 mg /L sulphate, 1.38-1.45 mg/L dissolved oxygen, 30.63-30.84 mg /L biochemical oxygen demand, 185.33-185.58 mg/L Ca, 84.68-84.83 mg /L Na,7.82-7.83 mg/L Fe, 0.35-0.36 mg/L Zn, 29.73-29.85 mg/L K, 0.51-0.52 mg /L Ni, 0.17-0.18 mg /L Cd and 0.42-0.43 mg /L Cr. The result revealed that the wetland is highly contaminated with cadmium. On comparison of the result obtained from the investigated wetland with other literature value there were variation in the physico-chemical properties of the water which may be attributed to change in temperature and in flow as the atmosphere changes. Most of the physicochemical parameters were within the standard limit set except for dissolved oxygen (DO) and biochemical oxygen demand (B0D) while majority of the trace metals were above the Standard Organization of Nigerian (SON) and World Health Organization (WHO) standard set limit.
Keywords: Wetland, Ecosystem, Biological cycle, Eutrophication, Aquatic fauna
Cite this paper: Bamgboye O. Agbeke, Osundiya M. Olubunmi, Olowu R. Adewale, Adeniyi David A., Physico-Chemical Analysis of Wetland Water of Iba Community in Lagos State Nigeria, American Journal of Chemistry, Vol. 6 No. 6, 2016, pp. 149-154. doi: 10.5923/j.chemistry.20160606.01.
Article Outline
1. Introduction
- Wetlands are defined as “areas of marsh, fen, peat land” or water, whether natural or artificial, permanent or temporary, with water that is stationary or flowing, fresh, brackish or salt, as well as areas of marine water, the depth of which at low tide does not exceed six meters and are the interface water and land [Ramsar (2006), USEPA (1995), Firehock et al (1998), NRC (1995)]. Wetlands covers 4-6% of the earth’s surface and are very productive ecosystems, which assist in the regulation of biological cycles, maintenance of water quality, nutrient movement and support for food chains. It provides important fish and wildlife habitat and also supports hunting, fishing and other recreational activities and the primary factor controlling the environment and the associated plant and animal life is water [Ramsar (2006), USEPA (1995)]. Wetland usually occur where the water table is at or close to the surface of land or anywhere the land is covered by shallow water beneath the text of their arms or convention [Ramsar (2006), USEPA (1995), NRC (1995)]. They possessed good biological diversity which makes the world productive environment [Mitsch, W.J. and J.G. Gosselink (1994), Mitsch, W.J. and J.G. Gosselink (1993), Kadlec R H, and Wallace S (2008)]. The domestic and industrial discharges into the wetlands will adversely affect the micro flora and fauna that dwell in them [Muwanga, A. and Banfago, I. (2006)] conversely, wetlands provide refuge for endangered species of plants and animals and economic benefits in aquatic fauna. They reduce the impact of floods by acting as storage areas [Muwanga, A. and Banfago, I (2006), Zhang B.Y (2010)]. Stored water percolates downward, getting purified in the process, and replenishing the ground water [Muwanga, A. and Banfago, I (2006)]. Interestingly, wetlands cover a tiny portion of the earth surface, but by the nature of their distinctive ecosystem, it becomes very important to protect and conserve them. Wetlands are important mechanism of watersheds and make available many important functions to the environment and to society at large [Mitsch, W.J. and J.G. Gosselink (1994), Mitsch, W.J. and J.G. Gosselink (1993), NRC (1995)]. However, as a result of the exponentially increasing demands of human expansion, and resource exploitation, urbanization and industrialization, natural wetland ecosystems are shrinking rapidly hence cannot always function efficiently for desired objectives and stringent water quality standards [Kadlec R H and Wallace S (2008), Wetzel, R G (1993)]. To assess the ecological nature of the wetlands physico-chemical quality of the wetland water can be investigated. It is a well known fact that discharges from domestic sewage and industrial effluent usually leads to changes in water quality and eutrophication. Several sources of water pollution which include mass bathing, rural waste matter, agricultural runoff and solid waste disposal has been reported by Wetzel, RG (1993), Olowu R. A et al (2010) and Adeniyi A.A et al (2007). In 2005, Rajar and his co worker investigated the physicochemical parameter of lakes wetland and reservoir in order to understand there water quality. Similar studies were also carried out by Jindal et a l 2007 and Kambele et al 2009 respectively. As a result this study is geared toward assessing the physicochemical parameter of wetland water around Iba community in Lagos state, Nigeria in order to ascertain the ecological nature of the wetland.
2. Materials and Methods
2.1. Study Location
- Iba village is situated within Iba Local Government Area of Lagos State. The Local Government share boundary with Ikotun-Igando Local Development area at the edge of the Obadore bridge to the north to include Iba community, to the south, Okokomaiko to the other side of Badagry expressway from Iyana-Iba up to the boundary of Oto-Awori Local Government, to the east, Iba road from Obadore bridge to the junction of Iba-Lasu road on Badagry expressway and to the west, Ketu bridge on Badagry expressway up to the boundary with Ogun State. In Iba village the wetland is a typical Bottomland Hardwood Forested Swamp wetland. It’s kind of wetland is characterized by a river (Odo-Isan), which flows through the village from inside Obadore village up to Ishasi and beyond. Over the years, the community have used and are still using the river as a means of transporting logs of wood from inside the swamp to a more open place where they are cut into pieces as firewood. Other activities engaged in by the people include fishing, hunting, palm wine taping, farming etc. The entire Iba village is a wetland, but in the recent years the wetland have been converted to various usage like construction of roads, construction of bridge, construction of drainage and building of houses. Despite these developments, wetlands still cover about 15 to 20 percent of the village landmass.
2.2. Sampling Collection and Preparation
- Water samples were collected for physico-chemical analysis from different sampling spots within the Iba wetland. Samples were collected, thrice, weekly for six weeks within a period of April 2010 –June 2010 in the morning between the hour of 6am-8am. The samples were collected in previously acid (5% HNO3) soaked and washed polypropylene. The samples in polypropylene bottles were refrigerated at 40 oC prior to all analysis procedures.The collected sample were analyzed for parameters such as (i) pH (ii) Total Suspended Solid (TSS) (iii) Chemical Oxygen Demand (COD) (iv) Alkalinity (v) Acidity (vi) Chloride (vii) Nitrites (viii) Phosphate (ix) Sulphate (x) Dissolved Oxygen (DO) (xi) Biochemical Oxygen Demand (BOD) (xii) Calcium (xiii) Sodium (xiv) Iron (xv) Zinc (xvi) potassium (xvii) Cobalt (xviii) Nickel (xix) Cadmium (xx) Chromium were analyzed in the laboratory following the standard methods of APHA, 2005. The trace metals were determined by a BUK scientific, VGP 210 model flame atomic absorption spectrophotometer.
3. Results and Discussion
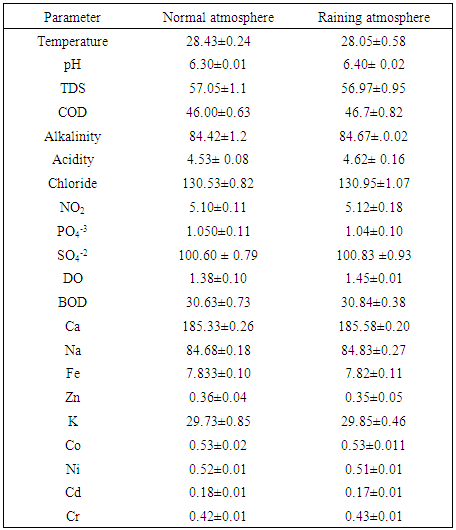
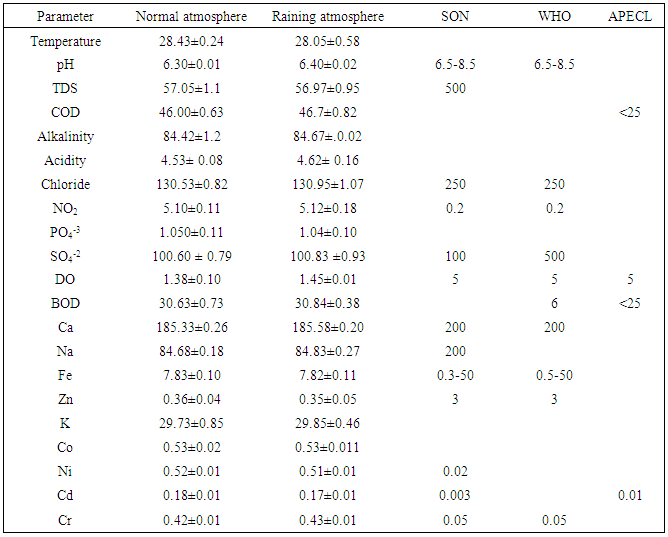
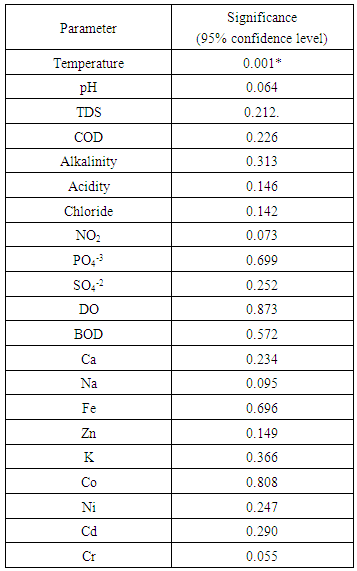
- Table 1 presented the average value and standard deviation of the wetland analysis results during normal and raining atmospheric conditions while figure 1 showed the variation between the two atmospheric conditions considered. The temperature of the water during the normal was 28.4 ◦C and 28.05 ◦C for raining atmospheric conditions. The average value of pH obtained was in the range of 6.30 ± 0.01 for the normal atmosphere and 6.40 ± 1.04 for raining atmospheric condition which revealed less acidic nature of the wetland. The value obtained for this wetland was lower than the value reported earlier by Kulakarni et al 2009 and the values are within the range recommended by standard organization of Nigeria (2007) of pH 6.5-8.5, suitable for drinking water SON (2007), Olowu et al (2010), WHO (1993), BIS (1991)]. On the order hand the chloride concentration obtained was in the range of 130.53 ± 0.82 mg/L for normal and 130.95 ±1.0 mg /L for raining conditions which was found to be higher than what was reported by Karnataka et al (2011) but was well within the allowed level of 200-250 mg/L recommended standard for portable water [SON (2007), Olowu et al (2010), WHO (1993), BIS (1991)] as shown in Table 1.
|
|
|
4. Conclusions
- Wetlands are important mechanism of watersheds and make available many important functions to the environment and to society at large. The physicochemical parameters and trace metal concentration level of the Iba wetland investigated was found to be highly polluted with trace metals most especially with cadmium when compared with standard limit set for portable water which may be attributed to various domestic discharges such as plastics, oils from generating sets as well as paint pigments and batteries used to power torchlight due to incessant power shortage in the area. The research work revealed that the wetland is highly polluted and hence is unsafe to be use as portable water for domestic usage. On the other hand the need to grow required plants that can absorbed the contaminants is very essential for phytoremediation of the Iba wetland is necessary so as to clean up the wetland for safety of the populate.
ACKNOWLEDGEMENTS
- The author wish to acknowledge the cooperation of the residents of the Iba community in Ojo local government of Lagos state Nigeria during sample collection.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML