-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2016; 6(5): 111-118
doi:10.5923/j.chemistry.20160605.01

Removal of Methylene Blue Dye and Lead from Water by Active Carbon from Mixed Sorghum and Millet Straws
A. O. Lawal, S. S. Achi, G. Wyasu, S. Usman, B. Okpara
Department of Chemistry, Kaduna State University, Nigeria
Correspondence to: A. O. Lawal, Department of Chemistry, Kaduna State University, Nigeria.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of this study is to determine the suitability of mixed straws of sorghum and millet as precursor for active carbon adsorbents with capabilities for removing dye and metal from water. Active carbons were produced from mixed straws of sorghum and millet by chemical activation with H3PO4. Mixed straws with particle size of 1180 µm were carbonized, in order to prevent low yield caused by low density and high ash content. The yield of the active carbon was 20% with pyrolysis temperature of 500°C. The potential of the active carbon as efficient and economical means of removing dye from solution was indicated by the removal of methylene blue from water samples while the carbon’s ability to remove metals from solution was indexed by aqueous phase adsorption of lead. The adsorption capacity of the active carbon for methylene blue was 10787mg/g. The adsorption data fitted the Freundlich isotherm and indicated strong adsorption of methylene blue to the active carbon. Equilibrium concentrations of lead were monitored, using Ultraviolet/Visible Spectrophotometer at a wavelength of 235 nm for EDTA-modified lead solution. The adsorption data also fitted the Freundlich isotherm. The Freundlich adsorption capacity of the granular active carbon for lead was 26.07mg/g with adsorption intensity of 0.7502. The results suggest the suitability of the carbon adsorbents for removal of lead and methylene blue dye from aqueous solution although the binding of Pb to the carbon adsorbent was weak.
Keywords: Active carbon, Sorghum, Millet, Straws, Methylene blue and lead
Cite this paper: A. O. Lawal, S. S. Achi, G. Wyasu, S. Usman, B. Okpara, Removal of Methylene Blue Dye and Lead from Water by Active Carbon from Mixed Sorghum and Millet Straws, American Journal of Chemistry, Vol. 6 No. 5, 2016, pp. 111-118. doi: 10.5923/j.chemistry.20160605.01.
Article Outline
1. Introduction
- Active carbon also referred to as activated carbon may be obtained from agricultural by-products and this offers an effective, low cost replacement for non-renewable coal-based granular active carbons [1]. The abundance and availability of agricultural by-products make them good precursors for active carbon production [2]. Alongside the environmental concern for dyes in wastewater, the removal of dye from wastewater by active carbon has been used to index the removal of organics from wastewater particularly in adsorption studies [3]. This is because of its known strong adsorption to active carbons. The water purification industry currently accounts for the large use of commercial active carbon. Drinking water and wastewater treatment plants utilize carbon filters to remove organics, micro-pollutants and metals by adsorption. The economics of the adsorption process greatly depends on the ability to reuse the active carbon. The problems associated with the use of active carbon adsorbents may be overcome by development of low cost adsorbents based on carbonaceous waste products that can be used on a once through basis and thus eliminating the need for regeneration.The Textile, paper and food industries, tanneries, electroplating factories are commonly associated with discharge of coloured wastewater [4]. Dyes are recalcitrant and persist for long distances in flowing water thereby retarding photosynthesis and growth of aquatic biota by blocking out sunlight. Dyes may cause allergic dermatitis, skin irritation, cancer and mutation. The techniques for removal of colour from industrial effluents include coagulation, floatation, biological treatment, hyper filtration, adsorption and oxidation. However, adsorption is generally the most preferred method and active carbon adsorbent is widely employed to treat the wastewater. Sawdust, coir pith, olive stone, pine bark, coconut shell, tropical grass and almond shells have been utilized as carbonaceous precursors for the removal of dyes from wastewater [5, 6].The production of active carbon in 1990 was estimated to be 375,000 ton, excluding the Eastern Europe and China [7]. In 2002, the demand for active carbon reached 200,000 ton per year in United States and the market is to be negatively affected by imports from Asia-Pacific region. The demands of active carbon were increased over the years and market growth was estimated at 4.6% per year [7, 8]. The strong market position held maintained by active carbon adsorbent relates to their unique properties and low cost compared with possible competitive inorganic adsorbents such as zeolites. The growth of the active carbon market may be sustained, considering the large number of raw materials available for the production of active carbon. In carbon production, dry oxidation methods involve the reaction with hot oxidizing gas such as steam and CO at temperatures above 700°C [6]. Wet oxidation methods involve the reaction between the carbon surface and solutions of oxidizing agents such as phosphoric acid (H3PO4), nitric acid (HNO3), hydrogen peroxide (H2O2), zinc chloride (ZnCl2), potassium permanganate (KMnO4), ammonium persulphate (NH4)2SO8 and potassium hydroxide KOH. Phosphoric acid and zinc chloride are commonly used for the activation of lignocellulosic materials [7]. Potassium hydroxide is usually used to activate coal or chars precursors. Zinc chloride has been reported to produce active carbon with higher specific area than that produced by phosphoric acid activation [8].However, phosphoric acid activation is widely preferred over zinc chloride because ZnCl2 has bad environmental impact and the active carbon produced cannot be used in food and pharmaceutical [9]. Chemical activation of carbon precursors has also been reported as more advantageous over physical activation due to higher yields, more surface area and better development of porous structures in carbon [10, 11].The use of active carbon usually increases the cost of treatment process. This drawback has stimulated the interest in utilization of cheap raw materials for the production of carbon adsorbent [12]. Consequently, a wide variety of agricultural by-products and wastes has been investigated as cellulosic precursors for active carbon in addition to hard wood and bituminous coal. The precursors include coconut shell and wood [13], Olive stones [14, 15], sugarcane bagasse, [16] pecan shells [17], palm seed, [18] apple pulp, [19] rubber seeds [20] and molasses [21]. Commercial active carbons are commonly produced from naturally occurring carbonaceous materials [22]. Due to the growing need for active carbons in the society and the high cost of raw materials, many researchers have attempted other waste materials such as tires, [23] resins, [24] agro wastes [25-31] and dried sewage sludge as raw materials and proposed new production methods [32-35] for active carbons with potential applications in pollution control. Furthermore, more interest has been devoted to utilize carbonaceous materials from paper mill sludge [36], old newspapers [37] and waste rubber [38]. Recently, activated sludge has been produced as a result of wastewater treatment activities and has emerged as an interesting option for the production of active carbon [39]. Chemical activation of sewage sludge with ZnCl2 and H2SO4 produced active carbon of high adsorption capacity comparable to commercial active carbon. The choice of a cheap precursor (waste) for the production of active carbon reduces the production cost and disposal problem.In the present study, agricultural waste materials, sorghum and millet straws were investigated for production of active carbon because of its availability in large quantity in sorghum and millet farms. The mixture of the milled straws was used to index the uses of the straws for carbon production without the need for sorting. The adsorption capacity of the active carbon was tested for aqueous adsorption of Pb and methylene blue dye.
2. Materials and Method
- All reagents were analytical grade and were used without further purification. UV/Visible spectrophotometric determination of Pb was accomplished by the formation of Pb-EDTA chelate which is more sensitive to UV/Visible detection. To prepare the stock solution, 0.186g Ethylenediamine Tetra-acetic acid (EDTA) and 1.0g Lead were weighed and dissolved with de-ionised water in 1000cm3 volumetric flask and made up to 1000ppm Pb-EDTA. 1 – 10ppm serial dilutions were prepared in a 100ml volumetric flask, using 0.1ml – 1.0ml of the stock solution. The absorbance of the Pb-EDTA solution was measured at 235nm using Jenway 6405 UV/VIS spectrophotometer.
2.1. Collection and Preparation of Precursors for Carbonization
- Dry straws of sorghum and millet were collected from Rigasa farm center (non industrial area) in Kaduna State (Nigeria), after harvest. The straws were cut into pieces of approximately 3 cm to obtain samples for milling. The chopped straw samples were milled with Christy and Morns miller, and sieved into a particle size of 1180µm as recommended by Lori et al., (2007). 0.25g each of sorghum and millet straws was mixed to produce the precursor for carbonization. 0.5g of the mixed straw powder was poured in five porcelain crucibles. To each of the samples, 2.5cm3 of H3PO4 was added as activating agent. The samples were heated for an hour at 110°C in the oven. The samples were transferred to furnace and were heated for an hour at 500°C after which the samples were washed using de-ionized water until the filtrates were neutral to litmus paper. The carbon residues were dried in oven at 110°C for one hour after which it was kept in the desiccator to cool and stored in an air tight container. Percentage yield of active carbon was calculated from,
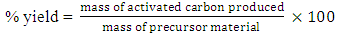
2.2. Methylene Blue Adsorption
- 0.1g active carbon was added to different concentrations (0.1-0.6ppm) of 100cm3 methylene blue solution in 250cm3 Erlenmeyer flasks. The flasks were sealed and kept for 24hours at normal room temperature of 27°C. After 24hours the mixtures were carefully filtered. The absorbance of the filtrates was measured using Jenway 6405 UV/Visible spectrophotometer at 645nm. The concentration of methylene blue adsorbed was calculated from the absorbance of the filtrate, using calibration curve. Methylene blue number (MBN) was calculated from the equation,
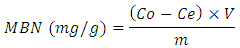 Where Co is initial concentration Ce is equilibrium concentration V is the volume of solution used m is the mass of active carbon used.
Where Co is initial concentration Ce is equilibrium concentration V is the volume of solution used m is the mass of active carbon used.2.3. Adsorption of Lead
- 0.1g of the prepared active carbon was placed in 250cm3 conical flasks. The respective Pb solutions (1, 2, 3, 4, 5, 6ppm) were added in each case and allowed to stand for an hour with intermittent shakings. The active carbon was filtered off and the filtrate was retained for analysis with UV/Vis spectrophotometer.
2.4. Freundlich Isotherm
- The Freundlich equation was employed for the adsorption of Methylene blue and lead on the adsorbent.The Freundlich isotherm was represented byqe= Kf Ce1/nThe linear form of the isotherm is,Log qe = log Kf + 1/n log CeThe Slope of the adsorption curve = 1/n, while Kf = 10interceptKf is a constant related to the adsorption capacity and 1/n is a constant indicating the adsorption intensity of the adsorbate onto the adsorbent.
3. Results and Discussion
3.1. Methylene Blue Adsorption
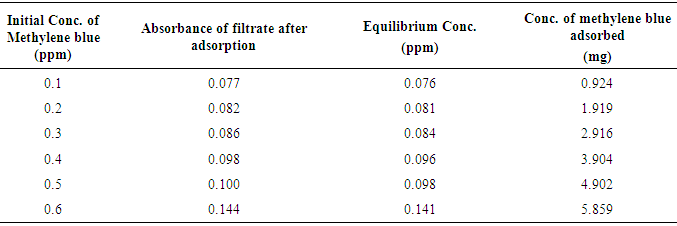
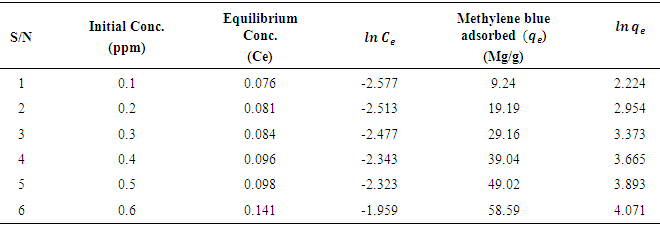
- The % yield of the active carbon from the mixed straws was 20%. Figure 1 is the calibration curve for methylene blue standard solution. The regression coefficient (R²) was 0.9602 and shows good linearity of the absorbance and concentrations of the methylene blue solutions with y = 1.018x. The equation of the line enabled the calculation of the concentration of the filtrate from the adsorption studies. The regression coefficient of determination indicates about 96% linearity. Tables 1 and 2 indicate that the equilibrium concentrations and amounts of methylene blue adsorbed on the active carbon increased with increase in the initial concentrations of the methylene blue solution.Figure 2 is the Freundlich isotherm for adsorption of methylene blue on the active carbon from mixed sorghum and millet straws. The adsorption data fitted the Freundlich isotherm. The Freundlich adsorption capacity of the granular active carbon from the cellulosic precursors was 10787mg/g with intensity of 2.5041. Figure 3 Shows the Freundlich adsorption intensity of methylene blue on active carbon produced from the mixed straws indicating adsorption constant of n = 0.4. This adsorption intensity index (n) is less than 1 and therefore describes a strong adsorption of methylene blue on the activated carbon. The results suggest the suitability of the carbon adsorbents for removal of methylene dye from aqueous solution.
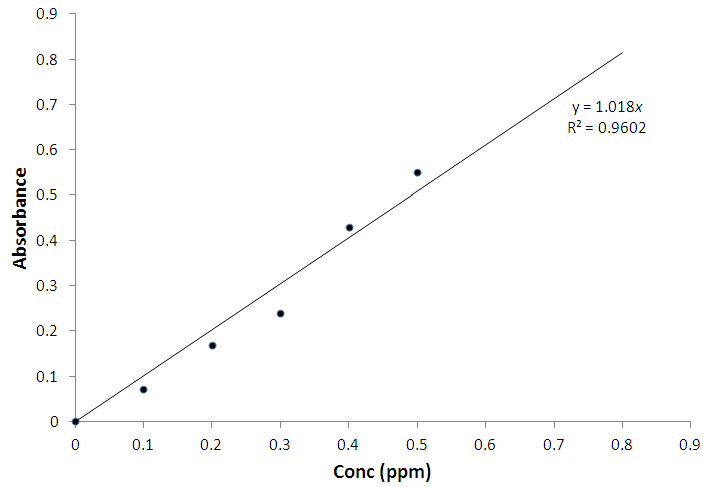 | Figure 1. Calibration curve for methylene blue |
|
|
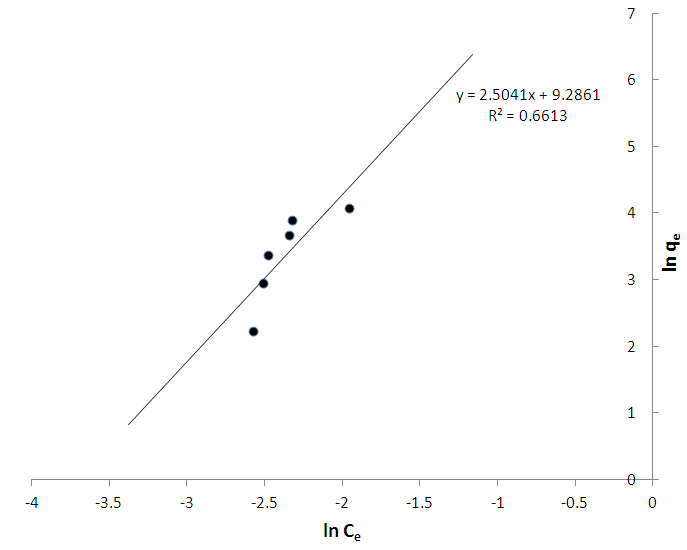 | Figure 2. Freundlich adsorption isotherm for adsorption of methylene blue on activated carbon from mixture of sorghum and millet straws |
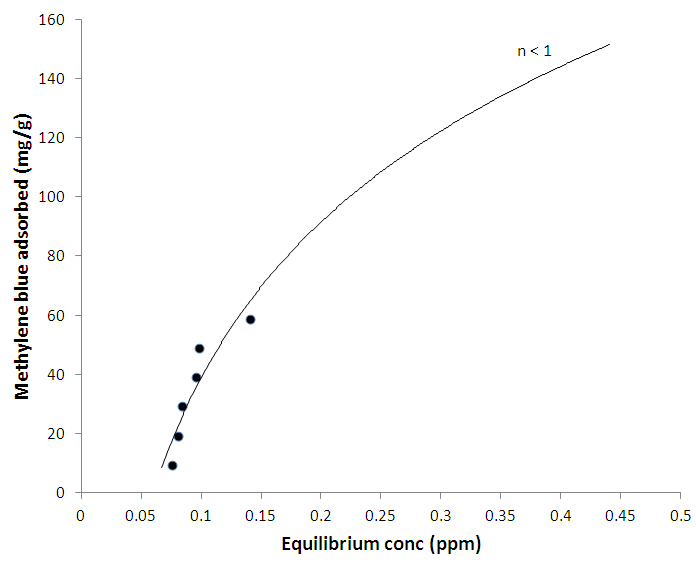 | Figure 3. Adsorption intensity of methylene blue on activated carbon from mixture of sorghum and millet straws |
3.2. Adsorption of Lead
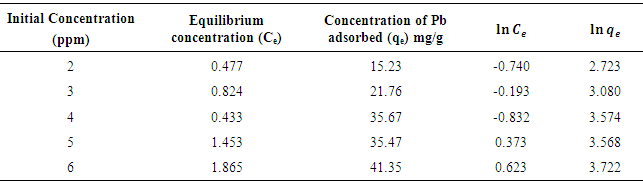
- Table 3 contains the adsorption data for the adsorption of Pb by the active carbon.
|
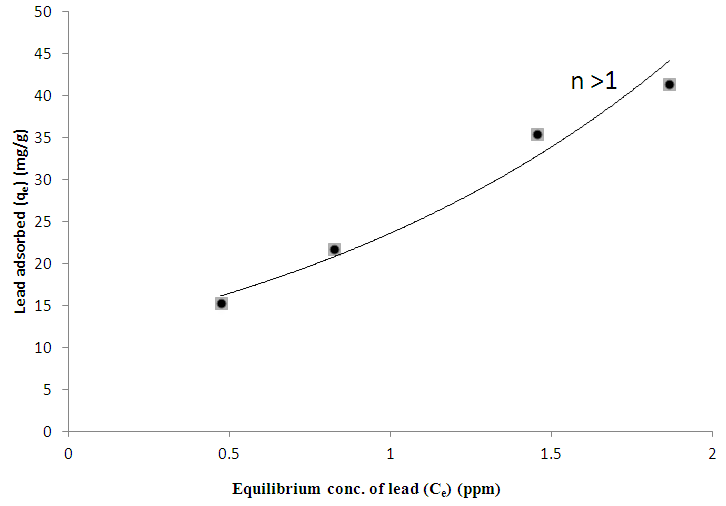 | Figure 4. Freundlich Adsorption intensity of lead on activated carbon from mixed sorghum and millet straws |
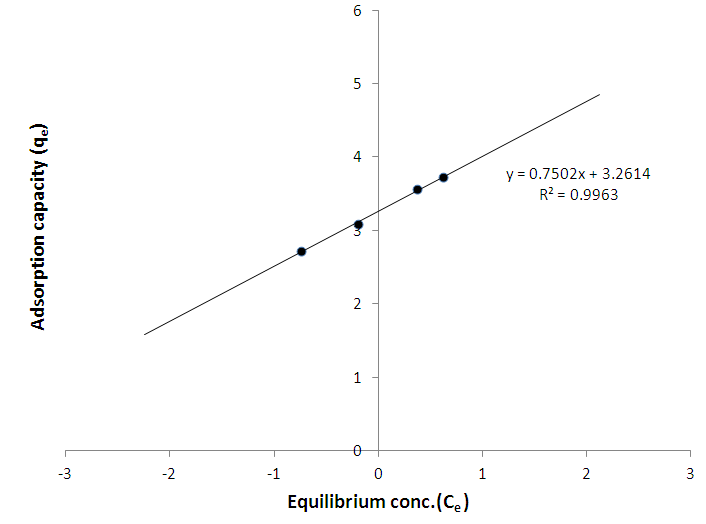 | Figure 5. Freundlich isotherm for adsorption of lead on activated carbon from mixture of sorghum and millet straws |
4. Conclusions
- Active carbon was prepared from mixtures of sorghum and millet straws. The maximum adsorption capacity of the active carbon for methylene blue is 10787mg/g. The results of this study suggest the suitability of active carbon from mixed straws of sorghum and millet for the removal dyes and metals from water. The active carbon indicated weak adsorption intensity and it has an adsorption capacity of 26.07mg/g for lead. Further investigation is required to study the removal mechanism of methylene blue by the activated carbon and describe the morphology of the carbon produced.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML