-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2016; 6(4): 104-110
doi:10.5923/j.chemistry.20160604.03

Novel Nanosized Mixed γ-Zirconium - Titanium Phosphates
S. K. Shakshooki, F. A. El-Akari, R. H. Idris, A. M. Hamassi, M. R. Eltalbi, Rabiea A. Elmabrouk, Amira A. Elfaki
Department of Chemistry, Faculty of Science, Tripoli University, Tripoli, Libya
Correspondence to: S. K. Shakshooki, Department of Chemistry, Faculty of Science, Tripoli University, Tripoli, Libya.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Novel nanosized γ-zirconium-titanium phosphates, γ-ZrxTi(1-x).PO4.H2PO4.2H2O (γ-ZTP), where x= 0.95, 0.90, 0.86, 0.80, were prepared and characterized by chemical, x-ray diffraction(XRD), thermogravimetric analysis(TGA) and Fourier transform spectroscopy (FT-IR). The resultant (γ-ZTP) found to be homogeneous solid solution forms. Their nanosized equal to 33.77, 40.64, 33.93 and 34.16 nm, respectively. Exchange capacities were determined and found to be in agreement with their formulation. These resultant γ-materials can be considered as new ion exchangers, solid acid catalysts, intercalates and as proton conductance.
Keywords: Nanosized, Mixed γ-zirconium - titanium phosphates
Cite this paper: S. K. Shakshooki, F. A. El-Akari, R. H. Idris, A. M. Hamassi, M. R. Eltalbi, Rabiea A. Elmabrouk, Amira A. Elfaki, Novel Nanosized Mixed γ-Zirconium - Titanium Phosphates, American Journal of Chemistry, Vol. 6 No. 4, 2016, pp. 104-110. doi: 10.5923/j.chemistry.20160604.03.
Article Outline
1. Introduction
- Tetravalent metal phosphates(TVMP) have been known for some time[1-4].Two dimensional (2D) layered tetravalent metal phosphates in α-, θ- or γ- forms, of general formulae, α-M(IV)(HPO4)2.H2O, θ-M(IV)(HPO4)2.5H2O, and γ-M(IV).PO4.H2PO4.2H2O, respectively, are well known [3-5], where M = Zr, Ti ,Hf, Sn, Ce. ..etc. Layered 2-D structures may be defined simply as solids where the bonds are strong between atoms within parallel planes in the structures, and much weaker between atoms in adjacent layers [4]. A single layer can therefore be regarded as a giant planar molecule. Usually atoms are covalently bonded within the layers, while weaker Van der Waals forces bond adjacent layers together. The distance between the bar centers of the layers is called the interlayer distance [3, 4]. Layered TVMP structures have been established [5, 6]. Tetravalent metal phosphates are very insoluble compounds with good thermal stabilities, and posses high ion exchange capacities [1, 2]. The synthetic method chosen for Layered (TVMP) plays a key role in determining the structural parameters of the resultant material [1-4].Applications of layered TVMP range from uses as sorbent in ion exchange [7, 8] and solid electrolytes [9] to catalysis [10]. The most extensively studies were on α-zirconium phosphate, α-Zr(HPO4)2.H2O(α-ZrP) [11, 12] and to some extent on α-titanium phosphate, α-Ti(HPO4)2.H2O(α-TiP) [13], the γ-compounds relatively less investigated [14, 15]. α-Titanium phosphate is isomorphs with α-zirconium phosphate. They found out gamma phase contain two chemically different types of phosphate groups, tertiary phosphate groups and dihydrogen phosphate groups [16]. In several fields of study, investigation into mixed metal compounds of increasing interest, as specific properties of single material can be improved by adding other. Mixed tetravalent metal phosphates in amorphous, glassy and in α-layered forms [17-22] have been prepared to improve certain properties of the single metal counter part. Mixed α-zirconium-titanium phosphates and α-hafnium-titanium phosphates of general formulae α-MxTi(1-x) (HPO4)2.nH2O are known [20, 22], where M= Zr, Hf, x= 0.9, 0.8, 0.67, 0.34, 0.2, 0.1 and n =1-2. However, to date mixed γ-zirconium-titanium phosphates are not known. Here we are reporting synthesis and characterization of novel nanosized mixed γ-zirconium-titanium phosphates, γ-ZrxTi(1-x) PO4.H2PO4 .2H2O, where x = 0.95, 0.90, 0.86, 0.80.
2. Materials and Methods
2.1. Chemicals
- ZrOCl2.8H2O, H3PO4 (85%), NH4.H2PO4 of (BDH), TiCl4 , HF(40%) of (Reidel De-Haen). Other reagents used were of analytical grade.
2.2. Preparation of γ-Zr0.95Ti0.05.PO4.H2PO4.2H2O (γ-Zr0.95Ti0.05 P)
- γ-zirconium-titanium phosphate ,γ-Zr0.95Ti0.05.PO4.H2PO4. 2H2O, was prepared by the reaction of a mixture 35 ml of 1.3M ZrOCl2.8H2O in 8M HF and 5ml 1M TiCl4 in 2.5M HF with 450 ml of 2M NH4H2PO4 , in plastic container at room temperature(~20°C) for 4 days. The resultant precipitate was filtered on Buchner funnel and subjected to washing with distilled water up to pH4, to obtain the monoammonium form γ-Zr0.95Ti0.05.PO4.NH4HPO4., that was converted to hydrogen form using 1M HCl with vigorous stirring at ~17°C for 24 h (for every 3g of γ-Zr0.95Ti0.05.PO4.NH4HPO4 , 600 ml 1M HCl were required). The resultant precipitate was washed with distilled water up to pH3, and was dried in air.
2.3. Preparation of γ-Zr0.9Ti0.1PO4.H2PO4.2H2O (γ-Zr0.9Ti0.1 P)
- γ-zirconium-titanium phophate, γ-Zr0.9Ti0.1.PO4.H2PO4. 2H2O phosphate , was prepared by the reaction a mixture of 35 ml of 1.3M ZrOCl2.8H2O in 8M HF and 10ml 1M TiCl4 in 2.5M HF with 450 ml of 2M NH4H2PO4 , in Pyrex round bottom flaks at room temperature(~20°C) for 4 days. The resultant precipitate was filtered on Buchner funnel and subjected to washing with distilled water up to pH4, to obtain the monoammonium form γ-Zr0.9Ti0.1.PO4.NH4HPO4. It was converted to hydrogen form using 1M HCl with vigorous stirring at ~17°C for 24h (for every 3g of γ-Zr0.9Ti0.1.PO4.NH4HPO4 600 ml 1M HCl were required). The resultant precipitate was washed with distilled water up to pH3, and was dried in air.
2.4. Preparation of γ-Zr0.86Ti0.14.PO4.H2PO4.2.11H2O (γ-Zr0.86Ti0.14 P)
- γ-zirconium-titanium phosphate ,γ-Zr0.86Ti0.14.PO4.H2PO4. 2.11H2O, was prepared by the reaction of a mixture 35 ml of 1.3M ZrOCl2.8H2O in 8M HF and 15 ml 1M TiCl4 in 2.5M HF with 450 ml of 2M NH4H2PO4 , in Pyrex round bottom flask at 35°C for 4 days. The resultant precipitate was filtered on Buchner funnel and subjected to washing with distilled water up to pH4, to obtain the monoammonium form -Zr0.86Ti14.PO4.NH4HPO4. It was converted to hydrogen form using 1M HCl with vigorous stirring at ~17°C for 24 h (for every 3g of -Zr0.86Ti14.PO4.NH4HPO4, 600 ml 1M HCl were required). The resultant precipitate was washed with distilled water up to pH3, and was dried in air.
2.5. Preparation of γ-Zr0.8Ti0.2.PO4.H2PO4.2.17H2O (γ-Zr0.8Ti0.2 P)
- γ-zirconium-titanium phosphate ,γ-Zr0.8Ti0.2.PO4.H2PO4.2.17H2O, was prepared by the reaction of a mixture 35 ml of 1.3M ZrOCl2.8H2O in 8M HF and 15ml 1M TiCl4 in 2.5M HF with 450 ml of 2M NH4H2PO4 , in Pyrex round bottom flask at 80°C with stirring for 24hours. The resultant precipitate was filtered on Buchner funnel and subjected to washing with distilled water up to pH4, to obtain the monoammonium form -Zr0.8Ti0.2.PO4.NH4HPO4. It was converted to hydrogen form using 1M HCl with vigorous stirring at ~17°C for 24h (for every 3g of -Zr0.8Ti 0.2.PO4.NH4HPO4, 600 ml 1M HCl were required). The resultant precipitate was washed with distilled water up to pH3, and was dried in air.The resultant mixed -ZTP were designated as compounds I, II, III, and IV, respectively. The preparation parameters carried out for compounds II, III and IV, shown above, at first the required amounts of a mixture of tetravalent metal salts were mixed with required amount of 2M NH4H2PO4 in plastic container with stirring for 15 minutes, then followed by transferring the mixture to Pyrex round bottle flask.
2.6. Estimation of Titanium Content
- Titanium content was determined spectrophtometerically as described earlier [2, 8].
2.7. Determination of the Exchange Capacities
- Exchange capacities of the resultant nanosized γ-ZrxTi(1-x) PO4.H2PO4 .2H2O were determined by addition of 25 ml of 0.10 M NaCl solution to100 mg of the resultant material, with stirring for one h, then titrated with 0.10 M NaOH solution.
2.8. Thermal Analysis
- Thermal analysis for the resultant materials were carried out under atmospheric condition, at rate of 10°C/min.
2.9. Instruments Used for Characterization
- X-ray PANalytical X,PertBRO, CuTarget (λ 1.545A45), TG/DTA SII Extra 6000 Thermogram. Fourier transform spectrometer, model IFS 25 FTIR- Bruker, pH Meter WGW.
3. Results and Discussion
- Novel nanosized γ-zirconium-titanium phosphates in homogeneous solid solutions forms., γ-Zr0.95Ti0.05..PO4.H2PO4.2H2O,γ-Zr0.9Ti0.1.PO4.H2PO4. 2H2O, γ-Zr0.86Ti0.14..PO4.H2PO4.2.11H2O, and γ-Zr0.8Ti0.2.PO4.H2PO4.2.17H2O, were prepared and characterized by chemical, x-ray diffraction(XRD), thermogravimetric analysis(TGA) and Fourier transform spectroscopy (FT-IR). Their titanium contents were determined spectrophotometically [2, 8], designated as compounds (I, II, III and IV), respectively.
3.1. XRD
- X-ray diffraction(XRD) is the principle technique used to characterize crystalline materials, and to determine unit cell size of a particular material.Figures(1-4) show (XRD) of the resultant nanosized γ-ZrxTi(1-x).PO4.H2PO4.2H2O(γ-ZTP), indicate good degree of crystallinity, where x= 0.95, 0.90, 0.86, 0.80, respectvely. Their interlayer distances (d001) found to be equal to 12.253, 12.238, 12.289 and 12.18 Å, respectively, The average diameter of γ-ZrxTi(1-x).PO4.H2PO4.2H2O, found to be equal to 33.77, 40.64, 33.93 and 34.16 nm, respectively, which were calculated from the full width at half maximum of the peak using Scherer’s equation:
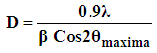 where D is the average crystal size in nm, λ is the characteristic wave length of x-ray used (λ=1.54056 Å), θ is the diffraction angle, and the β2θ is the angular width in the radius at intensity equal to half of the maximum peak intensity [23].Their XRD show the resultant products are in homogeneous solid solution forms [2, 24]. There is no indication of presence of pure α- or γ-titanium phosphates. The (d001) values of α- or γ-TiP in pure forms are 7.56 Å and 11.60 Å, respectively.
where D is the average crystal size in nm, λ is the characteristic wave length of x-ray used (λ=1.54056 Å), θ is the diffraction angle, and the β2θ is the angular width in the radius at intensity equal to half of the maximum peak intensity [23].Their XRD show the resultant products are in homogeneous solid solution forms [2, 24]. There is no indication of presence of pure α- or γ-titanium phosphates. The (d001) values of α- or γ-TiP in pure forms are 7.56 Å and 11.60 Å, respectively.3.2. TGA
- Figures (5-8) show the thermograms of the resultant nanosized γ-Zr0.95Ti0.05..PO4.H2PO4.2H2O, γ-Zr0.9Ti0.1. PO4.H2PO4.2H2O, γ-Zr0.86Ti0.14..PO4.H2PO4.2.11H2O, and γ-Zr0.8Ti0.2 .PO4. H2PO4 .2.17H2O, respectively, found to follow almost the same trend. Their total weight losses found to be, 17.02, 17.1, 17.62 and 17.72% in wt., respectively. The water of crystallization (~ two moles) are lost at ~100°C. At higher temperature condensation of POH groups occurs resulting in formation of [ZrxTi(1-x)]P2O7 as final product, the total loss was ~ three molecules of water per unit formula.Thermal decomposition of resultant γ-compounds (I, II) found to occur in three stages, accompanied by three endothermic peaks. The first two stages are related to the loss of water of crystallization, while the second stage is concern with the POH groups condensation. Thermal decomposition of resultant γ-compound (III) found to occur in three stages, accompanied by three endothermic peaks. The first stage is related to the loss of water of crystallization, while the second and third stages are concern with the POH groups condensation. Thermal decomposition of resultant γ-compounds (IV) found to occur in two stages, accompanied by two endothermic peaks. The first stage is related to the loss of water of crystallization, while the second stage is concern with the POH groups condensation.
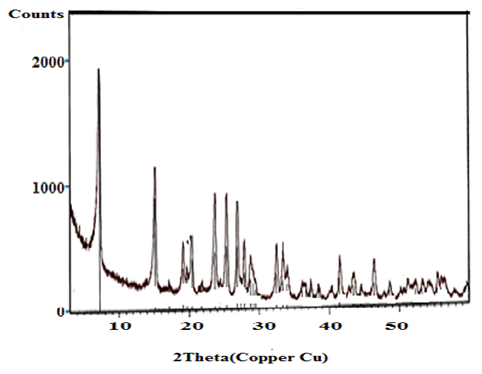 | Figure 1. XRD of γ-Zr0.95Ti0.05P |
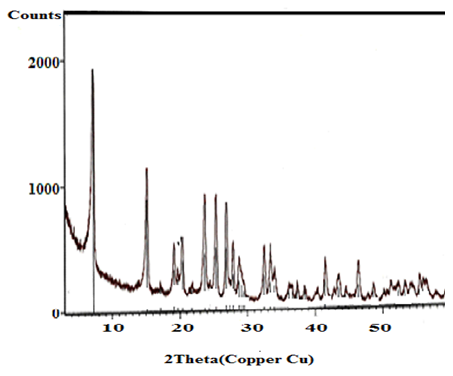 | Figure 2. XRD of γ-Zr0.9Ti0.1P |
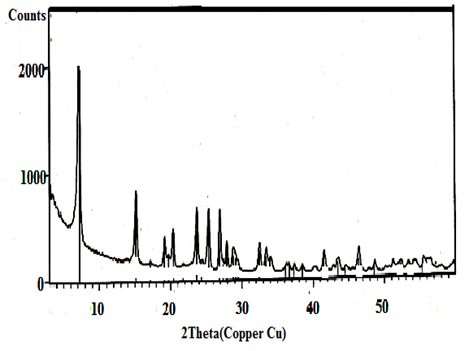 | Figure 3. XRD of γ-Zr0.86Ti0.14P |
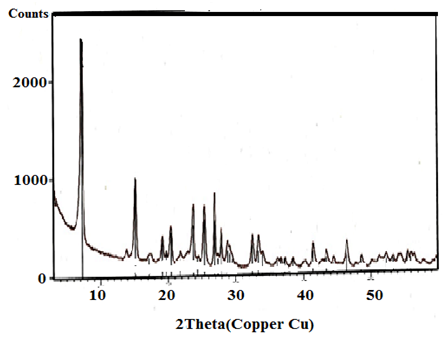 | Figure 4. XRD of γ-Zr0.8Ti0.2P |
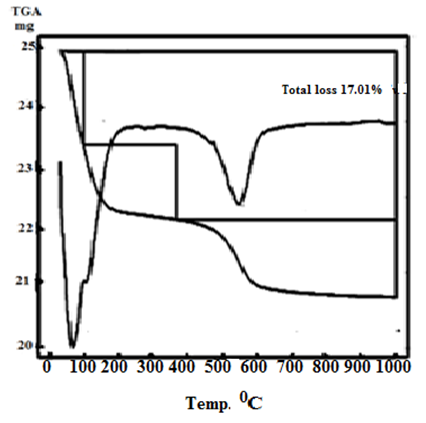 | Figure 5. TG/DTA of γ-Zr0.95Ti0.05P |
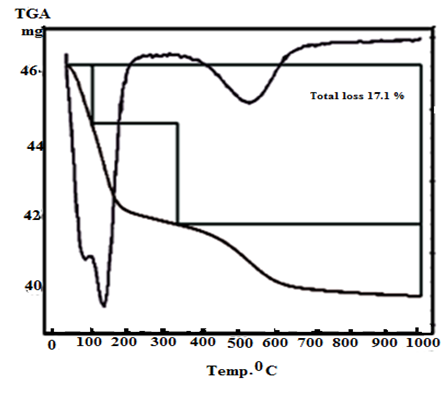 | Figure 6. TG/DTA of γ-Zr0.9Ti0.1P |
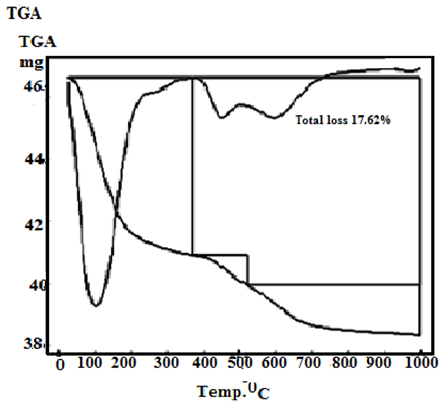 | Figure 7. TG/DTA of γ-Zr0.86Ti0.14P |
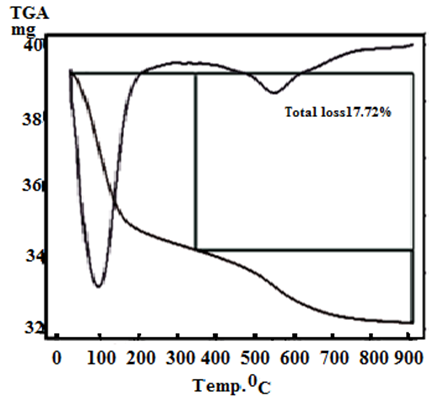 | Figure 8. TG/DTA of γ-Zr0.8Ti0.2P |
3.3. FT-IR
- Fourier transform infrared spectroscopy (FT-R) is well-established technique to obtain chemical composition by identifying specific bond structure of the studied material.Figures (9-12) show the FT-IR spectra of γ-Zr0.95Ti0.05..PO4.H2PO4.2H2O,γ-Zr0.9Ti0.1. PO4.H2PO4.2H2O, γ-Zr0.86Ti0.14..PO4.H2PO4.2.11H2O, and γ-Zr0.8Ti0.2 .PO4. H2PO4 .2.17H2O, respectively, with a trend similar to the IR spectra of γ-MIV phosphates [2, 8, 24]. Broad band centered at ~3350cm-1 is due to OH groups symmetric-asymmetric stretching of H2O, small sharp band at ~1630cm-1 is due to H-O-H bending, sharp broad band centered at ~1035cm-1 is related to phosphate groups vibration. The bands at the region 630-515 cm-1 are ascribe the presence of δ(PO4).
3.4. Exchange Capacities
- Exchange capacities of the resultant nanosized γ-ZrxTi(1-x). PO4.H2PO4 .2H2O (γ-ZTP), were determined by titration method as described in experimental section, found to be equal to 6.3, 6.33, 6.34 and 6.39 meq/g, respectively. Figures (13-16) show the Na+ ions titration curves of nanosized mixed γ-zirconium-titanium phosphates, γ-Zr0.95Ti0.05Zr0.95Ti0.05..PO4.H2PO4.2H2O,γ-Zr0.90Ti0.10..PO4. H2PO4.2H2O, γ-Zr0.86Ti0.14. .PO4. H2PO4.2.11H2O, and γ-Zr0.80Ti0.20 .PO4. H2PO4 .2.17H2O, respectively, found to follow same trend of Na+ ions titration curves of γ-metal (IV) phosphates [14, 15].
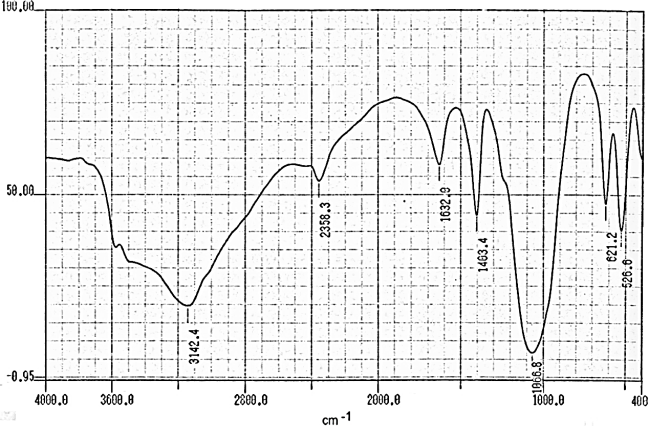 | Figure 9. FT-IR of γ-Zr0.95Ti0.05P |
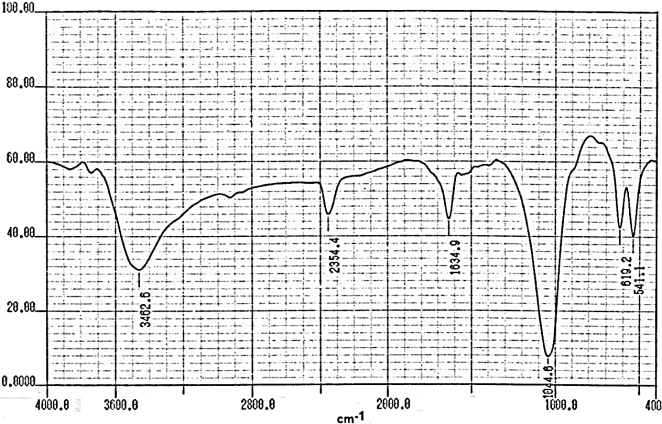 | Figure 10. FT-IR of γ-Zr0.9Ti0.1P |
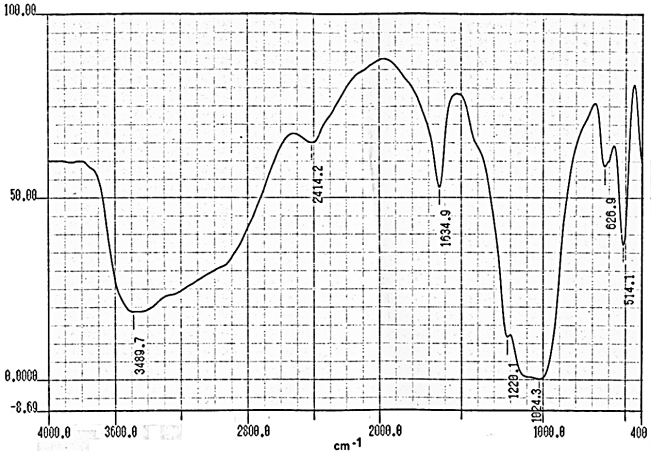 | Figure 11. FT-IR of γ-Zr0.86Ti0.14P |
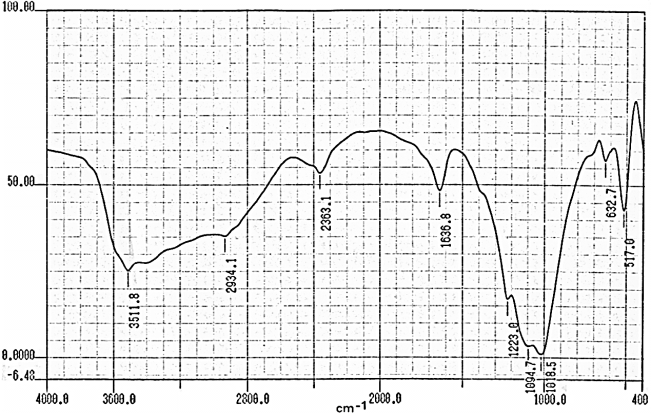 | Figure 12. FT-IR of γ-Zr0.8Ti0.2P |
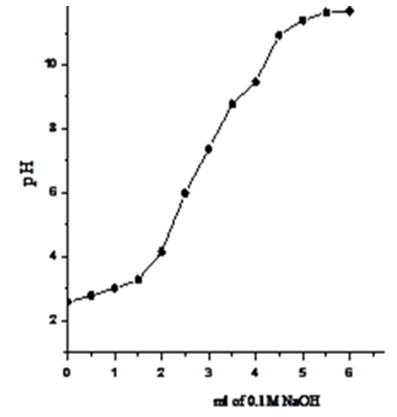 | Figure 13. Na+titration curve of γ-Zr0.95Ti0.05P |
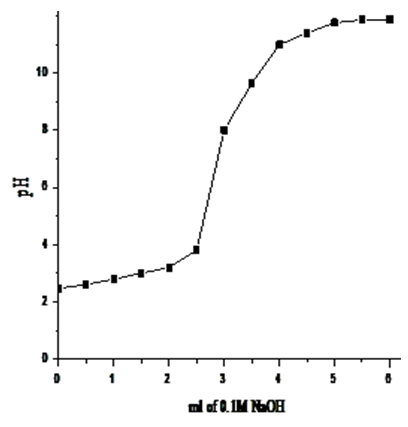 | Figure 14. Na+titration curve of γ- Zr0.9Ti0.1P |
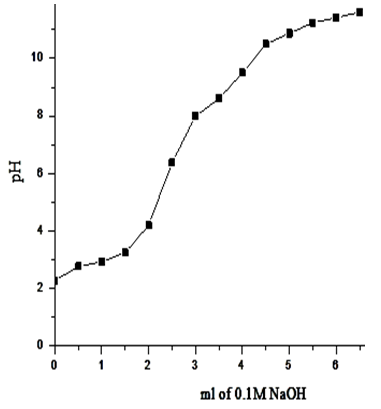 | Figure 15. Na+titration curve of γ-Zr0.86Ti0.14P |
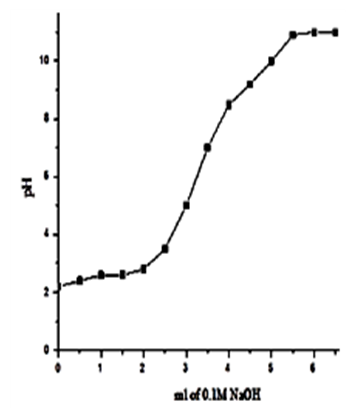 | Figure 16. Na+titration curve of γ-Zr0.8Ti0.2P |
4. Conclusions
- Novel nanosized mixed γ-zirconium–titanium phosphates, γ-Zr0.95Ti0.05..PO4.H2PO4.2H2O, γ-Zr0.9Ti0.1..PO4.H2PO4. 2H2O, γ-Zr0.86Ti0.14..PO4.H2PO42.11H2O and γ-Zr0.8Ti0.2.PO4. H2PO4 .2.17H2O, were prepared and characterized. Their XRD show the resultant products are in homogeneous solid solution forms. There is no indication of presence of α- or γ-titanium phosphates in pure forms. Their nanosized found to be equal to 33.77, 40.64, 33.93 and 34.16 nm, respectively. The titanium contents were determined. The nanosized (γ-ZTP) were formulated according to their chemical, XRD, TGA analysis and the estimation of their titanium contents. Their exchange capacities were determined and found to be almost in agreement to the theoretical values. These novel nanosized materials can be considered as new ion exchangers, solid acid catalysts, intercalates and as proton conductance.
ACKNOWLEDGEMENTS
- To Department of Chemistry, Faculty of Science, Tripoli University for providing facilities of research. To Geol. Adel Bayuomi, Institute of mineral resources, Egypt, for providing facilities of XRD analysis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML