-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2016; 6(4): 95-103
doi:10.5923/j.chemistry.20160604.02

Spectrophotometric Determination of Cobalt (II) with 2, 6-Dithiolphenol and Its Derivatives in the Presence of Hydrophobic Amines
Kerim A. Kuliyev, Nailya A. Verdizadeh, Geysar S. Suleymanova
Department of Chemistry, Azerbaijan State Pedagogical University, Baku, Azerbaijan
Correspondence to: Kerim A. Kuliyev, Department of Chemistry, Azerbaijan State Pedagogical University, Baku, Azerbaijan.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

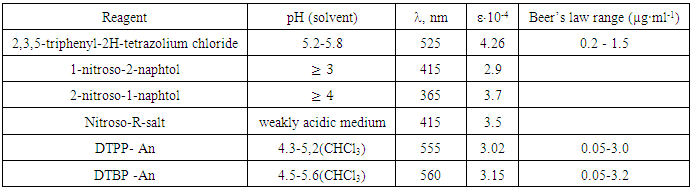
Mixed-ligand complexes of Cobalt (II) with dithiolphenol (DP) {2, 6-dithiolphenol (DTP) and its derivatives (2, 6-dithiol-4-methylphenol (DTMP), 2, 6-dithiol-4-propylphenol (DTPP), 2, 6-dithiol-4-tert-buthylphenol (DTBP)} in the presence of hydrophobic amines (Am) have been studied by spectrophotometry. Extraction of mixed ligand complexes is maximal at pH 4.1-5.6. The optimal conditions for the formation and extraction of mixed-ligand compounds have been found and the ratios of components I the complexes have been determined. The Beer’s law was applicable in the range of 0.05-3,2μg/ml. The effect of foreign ions and reagents on the extraction was studied. The method is free from common interferences. A procedure has been developed for extraction – spectrophotometric determination Cobalt in different objects.
Keywords: Cobalt, Solvent extraction, Spectrophotometry, Ion-associate
Cite this paper: Kerim A. Kuliyev, Nailya A. Verdizadeh, Geysar S. Suleymanova, Spectrophotometric Determination of Cobalt (II) with 2, 6-Dithiolphenol and Its Derivatives in the Presence of Hydrophobic Amines, American Journal of Chemistry, Vol. 6 No. 4, 2016, pp. 95-103. doi: 10.5923/j.chemistry.20160604.02.
Article Outline
1. Introduction
- Cobalt is a transition element of high industrial importance because of its valuable alloying, dyeing, magnetic, catalytic and plating properties. It is also of biological significance thanks to its ability to be an active center of coenzymes, e. g. vitamin B12. [1, 2]A great variety of photometric reagents is known for the determination of cobalt. However, the studies aiming to find and investigate new photometric reagents with different functional groups are still going on. For photometric determination of cobalt are quite selective reagents o-nitrozofenole group or a similar structure with the oxime group [3].The synthesis and characterization of mixed ligand complexes of Cobalt (II) with phthalic and heterocyclic amines were synthesized and characterized on the basis of elemental analysis, conductometric, magnetic measurements, UV-vis and IR spectral studies [4].The application of ternary and multicomponent complexes in spectrophotometric and spectrofluorimetric determination of trace elements is reviewed. Newer types of colour systems employing mixed ligand, surfactant sensitized, ion-association, flotation, derivative and FIA systems are described. Separate sections are devoted to advances in both spectrophotometric and spectrofluorimetric determination of individual elements. Future trends in spectrophotometric and spectrofluorimetric analysis are discussed [5].Complex formation and liquid-liquid extraction were studied in systems containing Co(II), 4-(2-pyridylazo) resorcinol, tetrazolium salt {2,3,5-triphenyl-2H-tetrazolium chloride (TTC) or 2-(4-iodophenyl)-3-(4-nitrophenyl) -5-phenyl-2H-tetrazolium chloride (INT)}, water and chloroform [6].The complex formation and a liquid-liquid extraction in the cobalt (II) - 4-(2- thiazolylazo) resorcinol (TAR) - 2,3,5-triphenyl-2H-tetrazolium chloride (TTC) - water – chloroform system was studied [7].Complex formation and liquid-liquid extraction were studied in a system containing cobalt(II), 4-(2-pyridylazo) resorcinol (PAR),1,4-diphenyl-3-(phenylamino)-1H-1,2,4- triazol [8, 9].A new solid-phase extraction method was developed for trace analysis of cobalt on Duolite XAD-761 resin by using flame atomic absorption spectrometry (FAAS) [10].Complex formation and liquid-liquid extraction were studied in a system containing cobalt (II), 4-(2-pyridylazo) resorcinol(PAR), 1,4-diphenyl-3-(phenylamino)-1H-1,2,4- triazole (Nitron, Nt), water, and chloroform. The effect of some experimental parameters (pH, shaking time, concentration of PAR, and concentration of Nt) was systematically investigated, and the optimum conditions for cobalt extraction as an ion-association complex, (NtH+) [Co3+(PAR)2], were found [11].Oxyphenolate and dithiophenolate complexes of cobaltare insoluble in chloroform, while mixed-ligand complexes with hydrophobic amines and aminophenols easily dissolve in various organic solvents [12-18].In this respect, a very promising reagent is dithiolphenols (DP), which contains one hydroxyl and two sulphohydryl groups and is a sulfur-containing analogue of mononuclear poly-phenols with two oxygen atoms replaced with sulfur atoms. The real work is devoted to studying of reaction of a complex formation of Cobalt (II) with dithiolphenoles (DP) in the presence of hydrophobic amines (Am). From dithiolphenols 2, 6-dithiolphenol (DTP), 2, 6-dithiol-4- methyl-phenol (DTMP), 2,6-dithiol-4-propylphenol(DTPP) and 2, 6-dithiol-4-tert-buthylphenol (DTBP) were used. in the presence of hydrophobic amines (Am). As hydrophobic amine aniline (An) and N-methylaniline (mAn). were used.
2. Experimental
2.1. Reagents and Apparatus
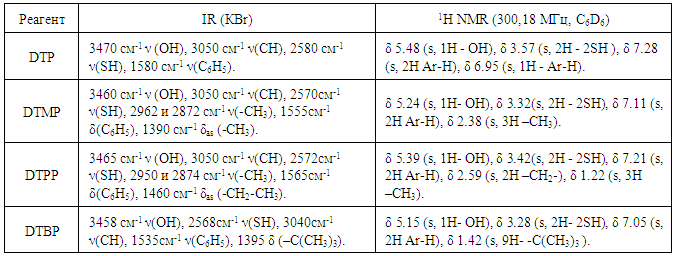
- A stock solution (1mg / mL) of cobalt (II) was prepared by dissolving in water an exact linkage CoSO4 • 7H2O in water containing 2 ml conc. H2SO4, and diluted with water to 1 liter [19]. The concentration of the cobalt solution was adjusted gravimetrically [20].Solutions of DP and Am in chloroform (0.01M) were used. DP were synthesized according to the procedure [21]. Their purity was verified by melting point determination and paper chromatography. The synthesized compound was identified by IR and NMR spectroscopy (table 1). To create the optimal acidity, 0.1M solutions of KOH and HCl or ammonium acetate buffers were applied. The extractant was purified chloroform.
|
2.2. General Procedure
2.2.1. General Procedure for the Determination of Cobalt (II)
- Portions of stock solutions of Cobalt(II) varying from 0.1 to 1.0 mL with a 0.1-mL step, a 2.2 mL portion of a 0.01 M solution of DP, and a 2.5 mL portion of a 0.01M solution of Am were placed in to calibrated test tubes with ground-glass stoppers (the volume of the organic phase was 5 mL). The required value of pH was adjusted by adding 1M HCl. The volume of the aqueous phase was increased to 20 mL using distilled water. In 15 minnute after the complete separation of the phases, the organic phase was separated from the aqueous phase and the absorbance of the extracts was measured on KFK-2 at room temperature and 440 nm (l=0.5cm).
2.2.2. Determination of Cobalt (II) in Steel
- A weighed sample of 0.2 g was dissolved in 20 ml of H2SO4 (1: 1) was oxidized with a few drops of concentrated nitric acid and evaporated twice lo vapor SO3. The precipitated salt was dissolved in 20 ml of 15% tartaric acid under heating, the solution was cooled, adjusted with water to 100 ml in a volumetric flask, stirred and filtered. An aliquot of 5 ml was put into a separatory funnel, was added 1 ml of 10% hydroxylamine solution, 1 ml of 3% ascorbic acid and was determined cobaltusing the proposed procedures.
2.3.3. Determination of Co (II) in Sewage Water and Bottom Sediments
- 1l taken for analysis of waste water is evaporated to obtain a precipitate, do not boil. The precipitate was dissolved in 5 ml of HNO3, was transferred to a 50 ml flask and diluted to the mark with water.
3. Results and Discussion
- Co(II) reacts with dithiolphenols (DP) and gives a yellow colored complexes. These complexes are insoluble in non-polar solvents. Experiments on electromigration in a U-shaped tube and on sorption on EDE-10P (EDE- ethylenediamine, epichlorohydrin; 10- serial number of the brand: P-means that the matrix has a macroporous structure) anion exchangers have demonstratedthe anionic nature of single-ligand complexes, in the electromigration study of the complexes, it was found that the yellow dithiophenolate complexes of Cobalt(II) moved to the cathode. When the sign of the charge of the single-ligand complexes was determined by ion chromatography, the EDE-10P anion exchanger completely absorbed the colored component of the solution. When hidrophob amins (Am) were introduced into the system, the extraction of these compounds into the organic phase as a mixed-ligand complex (MLC) was observed.
3.1. The Choice of the Extractant
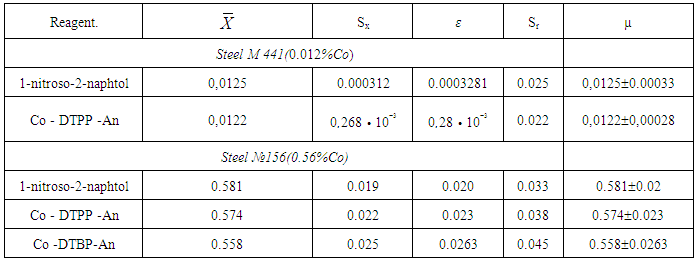
- For the extraction of complexes we used CHCl3, CCl4, C6H6, C6H5CH3, C6H4(CH3)2, C2H4Cl2, isobutanol and isopentanol. The extractivity of the complexes was estimated by the distribution coefficient and recovery. Chloroform, dichloroethane and chlorobenzene appeared to be the best extractants. All the further investigations were carried out with chloroform. The concentration of Cobalt in the organic phase was determined with 2-nitroso-1-naphtol [19] by photometric measurements after back extraction, while in the aqueous phase it was determined by the difference. The basicity of Am hardly influences the recovery of niobium. After a single extraction with chloroform, 96.9-99.2% of cobalt was extracted as an ion associate.
3.2. Influence of the pH of the Aqueous Phase
- The effect of pH on the formation of Co(II)-DP-Am complex was studied, in order to find a suitable pH that can be adopted in the determination of cobalt(II) (Fig. 1). The absorbance was found to be maximum in the pH range 4.1-5.6. Hence further analytical inves-tigations were carried out in media of pH 3. Extraction of Co(II) enhanced with the increase in the acidity of the initial solution; the further increase in acidity lead to the gradual decrase of recovery, which was obviously associated with a decrease in the concentration of the ionized form of DP. Probably, it is present in the solution in the non-dissociated state. At pH ≥ 8, the complexes were hardly extracted, obviously because of the decrease in the degree of Am protonation.
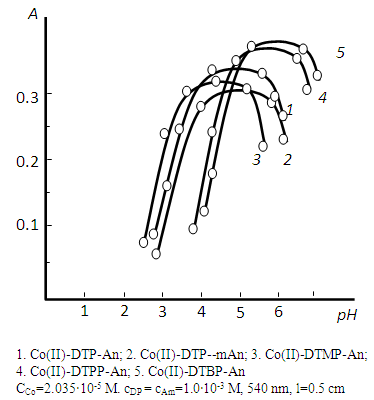 | Figure 1. Absorbance of mixed-ligand complexes as a function of the pH of the aqueous phase |
3.3. Influence of Reagent Concentration and Incubation Time
- For the formation and extraction of MLC, a 20-25-fold excess of complexing reagents is required; for example, the optimal conditions for formation and extraction of these compounds are provided by 1.0x10-3 M DP and (0.92-1.24) x 10-3 M Am. A large excess of hydrophob amin interferers with the determination. However it was found that the presence of excess of the reagent solution does not alter the absorbance of the color reaction. Unlike single-ligand complexes, mixed-ligand complexes of Co(II) with DP and Am were stable in aqueous and organic solvents and did not decompose for two days, or over a month after extraction. The required duration of the phase contact was 10 min.
3.4. Electronic Absorption Spectra
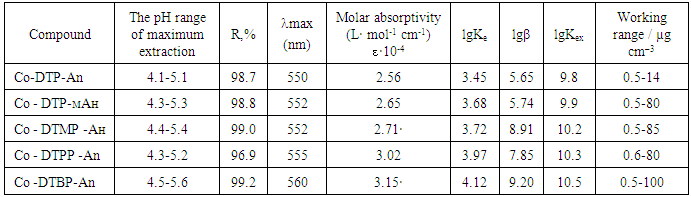
- Neither the metal ion nor the reagent has appreciable absorbance at specified wave-lengths. Hence further studies were carried out at 550-560 nm (fig.2). The reagent has minimum absorbance at the maximum absorbance of the complex. Hence further absorbance measurements were made at 540 nm. The molar absorptivity of the complex was calculated with Komar method [22] to be ε= (2.56-3.15) x 104 L mol-1 cm-1.
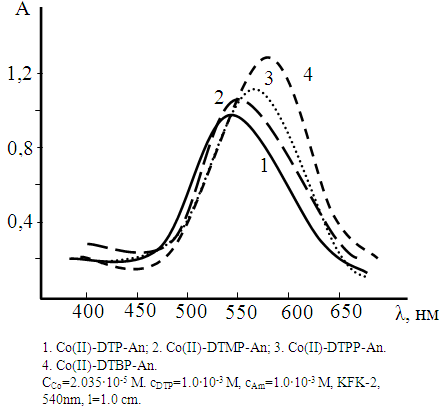 | Figure 2. Absorption of mixed-ligand complexes |
3.5. Stoichiometry of the Complexes and the Mechanism of Complexation
- Starik-Barbanel relative yield method, equilibrium shift method, crossed lines method and Asmus’ methods were employed to elucidate the composition of the complex [22]. The results suggest the complex composition of 1:2:2 (Co: DP: Am). The formation of MLC can be presented in the following way. When cobaltion interact with two molecules of DP, they form doubly-charged anionic complexes, which are extracted with two molecules of protonated Am. (Fig. 3). Formed ion-association complex between anionic chelates of cobalt (II) with DP and hydrophobic aromatic amines. The stability constant of Co(II)-DP-Am complexes was calculated and found to be lgβ = 7.19-11.25 at room temperature.
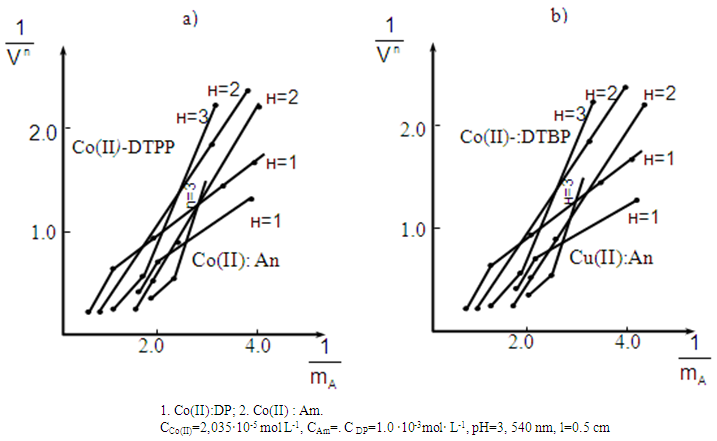 | Figure 3. Determination of the ratio of components by method of Asmus for (a) Co(II)-)-DTPP-An and (b) Co(II)-DTBP-mAn |
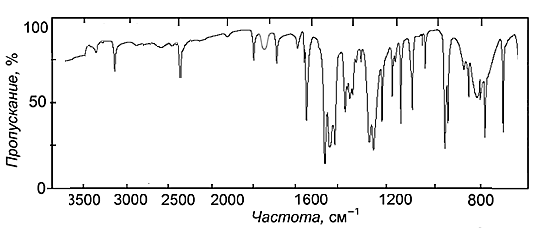 | Figure 4. IR spectrum of the complex Co(II)-DTPP-An |
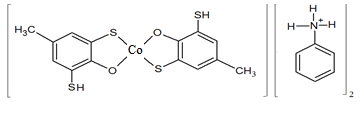 | Figure 5. Structure of complex [Co(DTMP)2](AnH)2 |
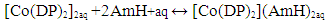 | (1) |
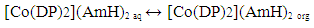 | (2) |
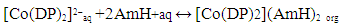 | (3) |
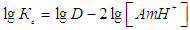 were presented in table 2.Calculation of extent of polymerization of complexes was carried out on the equation [27]. The made calculations showed that MLC in an organic phase won't be polymerized and are in a monomeric form (=1,05-1,12).
were presented in table 2.Calculation of extent of polymerization of complexes was carried out on the equation [27]. The made calculations showed that MLC in an organic phase won't be polymerized and are in a monomeric form (=1,05-1,12).3.6. Influence of Interfering Ions
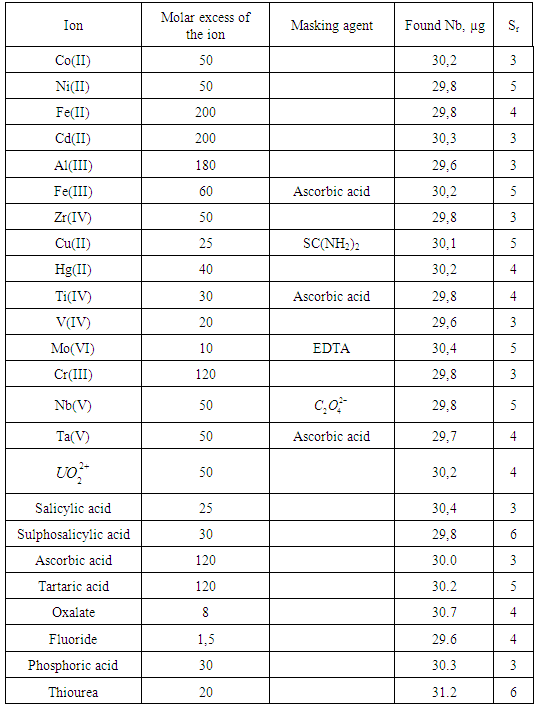
- The effect of various ions and reagents on the extraction-spectrophotometric determination of 5 mg cobalt (II) is summarised in Table 2. It can be assumed that large amounts of alkaline ions, alkaline-earth ions, NH4+, Cl–, S2O32–, F–, NO3–, SO42–, ClO4-, PO43–, tartrate, citrate, oxalate and tiron; moderate amounts of Cr(VI), Cr(III), Zn(II) and Cd(II); and small amounts of Mn(II), Sn(II), Cu(II), Al(III), ascorbic acid and SCN– are tolerable. Ni(II), Fe(II,III), V(IV,V), W(VI), Mo(VI), Ti(IV) and Nb(V) interfere determination of Co(II). However, the interfering effect of some of these ions can be reduced by masking with oxalate, citrate or EDTA (see Table 2).
|
|
3.7. Effect of Cobalt(II) Concentration
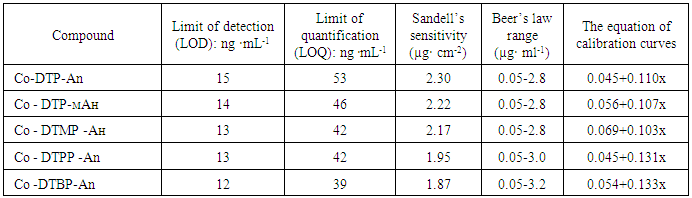
- The adherence to Beer’s law was studied by measuring the absorbance value of the series of solutions containing different concentrations of the metal ion. A linear calibration graph drawn between absorbance and the metal ion concentration indicates that Co(II) may be determined in the range 0.05-3.2 μg/ml (table 4). The pertaining calibration graph is shown in the Fig. 6.
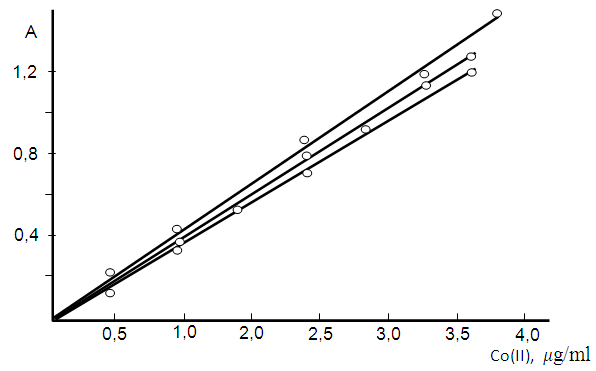 | Figure 6. Analytical determination of Co (II); 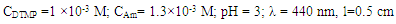 |
|
|
3.8. Analytical Applications
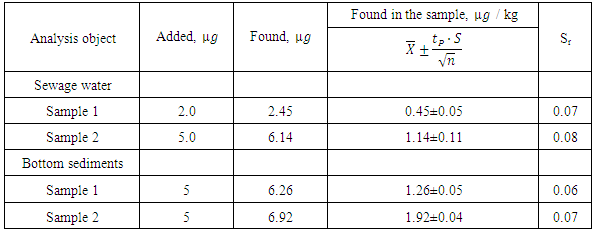
- The proposed method under the already established optimum conditions was applied for the determination of Co(II) in various objects. The results presented in Table 6 and Table 7 indicate the successful applicability of the proposed method to real sample analysis.
|
|
3.9. Correlation between Properties of the Reagents and Complexes
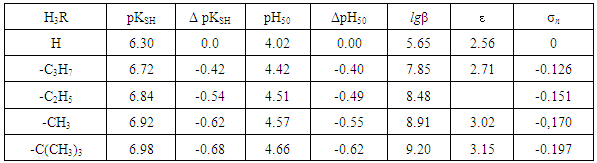
- To establish relationships between the acid-base properties of the sulfhydryl group (pKSH) and some properties of the ternary complexes (ΔpH50 and lgβ) we constructed Fig. 7 and Fig. 8. The pH50 values were determined graphically from the dependence A=f(pH) (see Fig. 1) for absorbance of 50% (Tabl. 8). ΔpKSH values in Fig. 7 are the differences between pKSH of the unsubstituted reagent (DTP) and pKSH of its substituted analogues (DTMP, DTPP, DTBP). ΔpH50 in Fig. 7. is the corresponding difference between the pH50 values for DTP and DTMP, DTPP or DTBP (R2=0.9985).
|
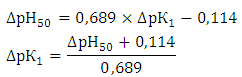
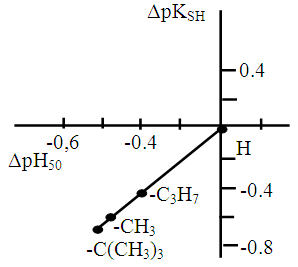 | Figure 7. Correlation between the acidic properties of DP (ΔpKSH) and ΔpH50 for the Co(II)-DP-An complexes |
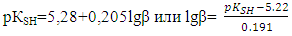
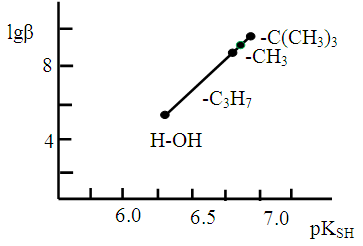 | Figure 8. Correlation between the acidic properties of DP (pKSH) and lgβ for the Co(II)-DP-An complexes |
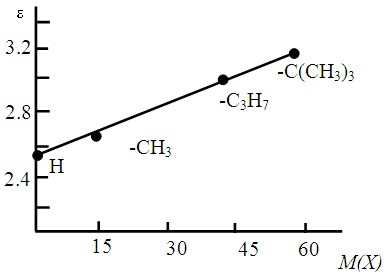 | Figure 9. Correlation between the molar mass (M(X)) and molar absorptivity of the Co(II)-DP-An complexes |
4. Conclusions
- 1. Mixed-ligand complexes of Cobalt (II) with DP and Am have been studied by spectrophotometry. Extraction of mixed ligand complexes is maximal at pH 4.1-5.6. The optimal conditions for the formation and extraction of mixed-ligand compounds have been found. 2. The molar ratio of the reacting Co(II), DP and Am species is 1:2:2. The general formula of the ternary complexes is [Co(DP)2] (AmH)2. They can be regarded as ion-associates between doubly charged anionic chelates [Co(DP)2]2- and protonated Am species. 3. The developed method retains specific interaction of cobalt (II) with DP and Am to form a colored complex and has good sensitivity at room temperature. The proposed method has significant advantage over the other spectrophotometric methods in terms of simplicity and sensitivity. This proposed method has good precision and accuracy. A procedure has been developed for extraction-spectrophotometric determination Cobalt in various objects.4. Relationships exist between the acid-base properties of the sulfhydryl group of DP (pK1) and some characteristics of the ternary complexes. The relationship between ΔpKSH and ΔpH50 for the Co(II) complexes can be adequately described by a straight-line equation
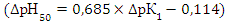 . The relationship between M(X) and εmax for the same complexes can be described by a linear regression equation (ɛ=2.56 +0.01M(X)).
. The relationship between M(X) and εmax for the same complexes can be described by a linear regression equation (ɛ=2.56 +0.01M(X)).  Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML