-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2016; 6(4): 87-94
doi:10.5923/j.chemistry.20160604.01

Synthesis and Characterization of Glassy Tin Phosphate/Polyindole, Polyaniline, Polypyrrole, Polyindole-co-Polyaniline, Polyindole-co-Polypyrrole Composites Via In-Situ Oxidative Polymerization
S. K. Shakshooki, A. A. Elejmi, Hanan, A. Emmsalem
Department of Chemistry, Faculty of Science, Tripoli University, Tripoli, Libya
Correspondence to: S. K. Shakshooki, Department of Chemistry, Faculty of Science, Tripoli University, Tripoli, Libya.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Glassy tin phosphate, Sn(HPO4)2.2.78H2O (SnP), was prepared and characterized by chemical, X–ray, thermal analysis and FT-IR spectroscopy. Its exchange capacity was determined and found to be equal to 5.1 Meq/g. Novel glassy tin phosphate/polyaniline, -/polypyrrole, -/polyindole, -/polyindole-co-polyaniline, -/polyindole-co-polypyrrole composites were prepared via in-situ chemical oxidative polymerization of the monomers: indole, aniline, pyrrole and co-monomers: indole-co-aniline, indole-co-pyrroleusing FeCl3 as oxidant. The resultant composites were characterized by elemental (C,H,N) analysis, FT-IR spectroscopy, and scanning electron microscopy(SEM). SEM images of the resultant composites reveal distribution of the polymers and co-polymers on the inorganic matrix. From elemental (C,H,N) analysis the amount of organic material present in SnP/PIn composite = 15.0% in wt, SnP/Pani composite = 3.298% in wt, SnP/PPy composite = 36.48% in wt., respectively. The amount of organic material present in SnP/PIn-co-Pani = 3.837, where (PIn = 0.54% in wt, Pani = 3.29% in wt). Organic material present in SnP/PIn-co-PPy found to be 41.725% in wt, where (PIn = 11.803% in wt, PPy = 30.023% in wt).
Keywords: Glassy tin phosphate, Oxidative, Polymerization, Indole, Aniline, Pyrrole, Indole-co-aniline and indole- co-pyrrole
Cite this paper: S. K. Shakshooki, A. A. Elejmi, Hanan, A. Emmsalem, Synthesis and Characterization of Glassy Tin Phosphate/Polyindole, Polyaniline, Polypyrrole, Polyindole-co-Polyaniline, Polyindole-co-Polypyrrole Composites Via In-Situ Oxidative Polymerization, American Journal of Chemistry, Vol. 6 No. 4, 2016, pp. 87-94. doi: 10.5923/j.chemistry.20160604.01.
Article Outline
1. Introduction
- Tetravalent metal phosphates are very insoluble compounds with good thermal stabilities, and posses high ion exchange capacities. They have been known as amorphous for some time, of general formula M(IV)(HPO4)2.3-5H2O [1, 2]. The discovery of their layered crystalline materials [3, 4], represent a fundamental step in chemistry of these compounds. Increase attention direct toward their intercalation [5, 6], catalytic [7], electrical conductance [8], and sensors [9]. Their layered crystalline materials, resemblance clay minerals, exist as two dimensional (2-D) and three-dimensional (3-D)structures [10]. Layered twodimensional structure exist in α, γ and θ forms of general formula α-M(IV)(HPO4)2.H2O, γ-M(IV).PO4.H2PO4.2H2O, θ-M(IV)(HPO4)2.5H2O, respectively [11-13] (where M = Ti, Zr, Hf, Ge, Sn , Ce ….etc). Conducting polymers containing nitrogen atoms like polyaniline and polypyrrole, and their substituted derivatives have elicited much interest among researchers because of their reasonably good conductivity, stability, ease of preparation, affordability and redox properties compared to other organic compounds. In particular, the electronic and electrochemical properties of conducting polymers have made them find applications in photovoltaic cells, organic light emitting diode, sensors and others [14-16]. Their electrical and electrochemical properties show great promise for commercial applications. A conducting polymer can act as a semiconductor or a conductor If all the available charge carriers of poly anilili sal [Emiraledine salt(ES)] Known as Pani, were to contribute, the resulting conductivity at room temperature would be ~ 105 S cm-1, which is comparable to that of copper [15]. The higher values of the electrical conductivity obtained in such organic polymers have led to the name ‘synthetic metals’.However, among various aromatic compounds-based conducting polymers polyindole and its derivatives has been less investigated although there exists close structural similarities with the polymers mentioned above [17]. Polyindole can be obtained from chemical, electrochemical and interfacial polymerization of indole [15-18]. Its electrical and electrochemical properties show great promise for commercial applications [16-20]. Polyindole is an electro active polymer, owns advantages especially fairly good thermal stability [18, 19]. Some studies shows polyindole has similar properties like polyaniline, based on their high conductance and good environmental stability [15, 17, 21, 22]. The properties of these materials depend strongly on their synthesis method [15, 18, 21, 23].Recently a great deal of attention has been paid towards synthesis of conducting co-polymers because the co-polymer provides higher properties than the individual conducting polymer. It has been observed experimentally that copolymerization of two different monomers increases the number of conductive polymers which cannot be achieved by using single monomer [28-33]. Conducting polymers and copolymers can be prepared via chemical [16, 18] or electrochemical polymerization [15, 29, 32].Tetravalent metal phosphates/conducting polymer composites comprise an important class of synthetic engineering. However; research in such area is still Terra incognita [34-36].
2. Materials and Methods
2.1. Chemicals
- SnCl4 , H3PO4 (85%) of BDH, aniline (99.5%) of Mindex UK., pyrrole of Aldrich, Indole of Reidel de-Haen. Other reagents used were of commercial analytical grade.
2.2. Instruments used for Characterization
- X-ray powder diffractometer Siemens D-500, using Ni-filtered CuKα (λ= 1.54056Å), TG/DTA SII Extra 6000 Thermogram, and Fourier Transform IR spectrometer, model IFS 25 FTIR, Bruker , pH Meter WGW.
2.3. Preparation of Glassy Tin Phosphate, Sn(HPO4)2.2.78H2O(SnP)
- Solution of 0.3M SnCl4 in 4M HCl were mixed with solution of 9M H3PO4 (1 : 2.4 molar ratio, respectively), with stirring at room temperature for 2h followed by adjusting the pH of the reactants by 1M NaOH up to pH2. The resultant tin phosphate gel was washed by distilled water up to pH3, filtered on Buchner funnel to give wet gel of tin phosphate cake that was dried at 60°C, then water cracked. On filtration, glassy tin phosphate of vitreous look, was obtained.
2.4. Determination of the Exchange Capacities of Glassy Tin Phosphate
- To 0.1g of tin phosphate, 25ml of 0.1M NaCl were added, stirred vigorously for 1hr, then titrated with 0.1M NaOH.
2.5. Thermal Gravimetric Analysis (TGA)
- Thermal analysis was carried at the range 21-800°C, under atmospheric condition , the heating rate was 10°C/min.
2.6. Preparation of Glassy Tin Phosphate/Polyindole Composite
- 0.25g SnP was dispersed in 5ml distilled water, with stirring for 15 minutes, at room temperature (20ºC), followed by the addition of 16 ml 4% indole in ethanol. The stirring was continued for an hour. To that 10ml 8% FeCl3 , was added drop wise, the stirring was continued for 24h at room temperature (20ºC). The resultant composite was filtered, washed with distilled water, and ethanol, and left to dry at room temperature.
2.7. Preparation of Glassy Tin Phosphate/Polyaniline Composite
- 0.25g SnP was dispersed in 5ml distilled water, with stirring for 15 minutes, at room temperature (20ºC), followed by the addition of 16 ml 4% aniline in ethanol. The stirring was continued for an hour. To that 10ml 8% FeCl3 , was added drop wise, the stirring was continued for 24h at room temperature (20ºC). The resultant composite was filtered, washed with distilled water, and ethanol, and left to dry at room temperature.
2.8. Preparation of Glassy Tin Phosphate/Polypyrrole Composite
- 0.25g SnP was dispersed in 5ml distilled water, with stirring for 15 minutes, at room temperature (20ºC), followed by the addition of 16 ml 4% pyrrole in ethanol. The stirring was continued for an hour. To that 10ml 8% FeCl3 , was added drop wise, the stirring was continued for 24h at room temperature (20ºC). The resultant composite was filtered, washed with distilled water, and ethanol, and left to dry at room temperature.
2.9. Preparation of Glassy Tin Phosphate/Polyindole-co-polyaniline Composite
- 0.25g SnP was dispersed in 5ml distilled water, with stirring for 15 minutes, at room temperature (20ºC), followed by the addition of mixture of 1:1 ratios, indole: aniline, (8 ml 4% indole: 8 ml 4% aniline) in ethanol. The stirring was continued for an hour. To that 10ml 8% FeCl3 , was added drop wise, the stirring was continued for 24h at room temperature (20°C). The resultant composite was filtered, washed with distilled water, and ethanol, and left to dry at room temperature.
2.10. Preparation of Glassy Tin Phosphate/Polyindole-co-polypyrrole Composite
- 0.25g SnP was dispersed in 5ml distilled water, with stirring for 15 minutes, at room temperature (20°C), followed by the addition of mixture of 1:1 ratios, indole: pyrrole, (8 ml 4% indole: 8 ml 4% pyrrole) in ethanol. The stirring was continued for an hour. To that 10ml 8% FeCl3 , was added drop wise, the stirring was continued for 24h at room temperature (20°C). The resultant composite was filtered, washed with distilled water, and ethanol, and left to dry at room temperature.
3. Results and Disscusion
- Glassy tin phospahte, Sn(HPO4)2.2.78H2O(SnP), was prepared and cahracterized by chemical X-ray diffraction(XRD), FT-IR spectroscopy and thermal analysis(TGA). Its exchange capacity was determined by Na+ ions potentiometric titration.
3.1. XRD of SnP
- Figure (1) shows X-ray diffraction (XRD) pattern of glassy tin phosphate, Sn(HPO4)2 .2.78H2O, found to follow similar trend of amorphous M(IV) phosphates[1, 2].
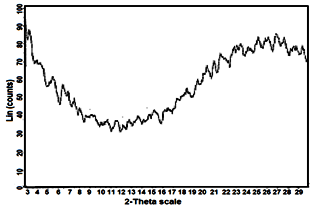 | Figure 1. XRD for glassy Sn(HPO4)2.2.78H2O |
3.2. Na+ ion Titration Curve of SnP
- Figure (2) shows the Na+ ions potentiometric titration curve of glassy tin phosphate, Sn(HPO4)2 .2.78H2O, which shows same trend of that of the Na+ ions titration curves of amorphous M(IV) phosphates [1, 2]. Its exchange capacity found to be equal to 5.1 Meq/g.
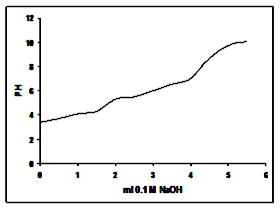 | Figure 2. Shows that Na+ ions titration curve of glassy Sn(HPO4)2.2.78H2O |
3.3. TGA of SnP
- Figure (3) shows the thermogram of glassy tin phosphate, Sn(HPO4)22.78H2O. Its thermal behaviour found to follow similar trend to that of amorphous M(IV) phosphates [1, 2]. The thermal decomposition found to occur in two steps, first step loss of water of hydration at the temperature range 80-230°C, followed by POH groups condensation up to ~650°C. Total wt% loss found to be equal to 13.65%. The final product was SnO2.P2O5.
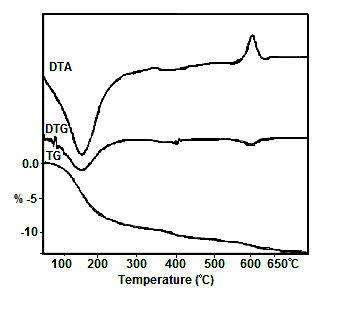 | Figure 3. TG/DTA/DTG of glassy Sn(HPO4)2.2.78H2O |
3.4. FT-IR of SnP
- Figure (4) shows FT-IR spectrum of glassy Sn(HPO4)2. 2.78H2O, which found to be typical to FT- IR spectra of amorphous M(IV) phosphates [1, 2]. Broad band centered at ~ 3470cm-1 is due to OH groups symmetric-asymmetric stretching of H2O, small sharp band at1625cm-1 is due to H-O-H bending, sharp broad band centered at 1050cm-1 is related to phosphate groups vibration.
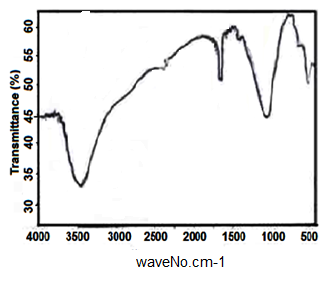 | Figure 4. FT-IR spectra of glassy Sn(HPO4)2 .2.78H2O |
3.5. SnP/Polyaniline Composite
- It was found when aniline in ethanol solution was added to slurry aqueous solution of glassy tin phosphate(SnP), with stirring , then followed by the addition of the oxidant FeCl3, to give bluish-green color by the end of the reaction period. The resultant SnP/ polyaniline composite was characterized by elemental (C, H, N) analysis, FT-IR spectroscopy and SEM. From elemental (C, H, N) analysis. The amount of polyaniline present in the composite was 3.298% in wt.
3.5.1. SEM of SnP/Polyaniline Composite
- Figure (5) shows SEM image for glassy, SnP/ polyaniline composite, reveal a distribution of the polymer on the inorganic matrix.
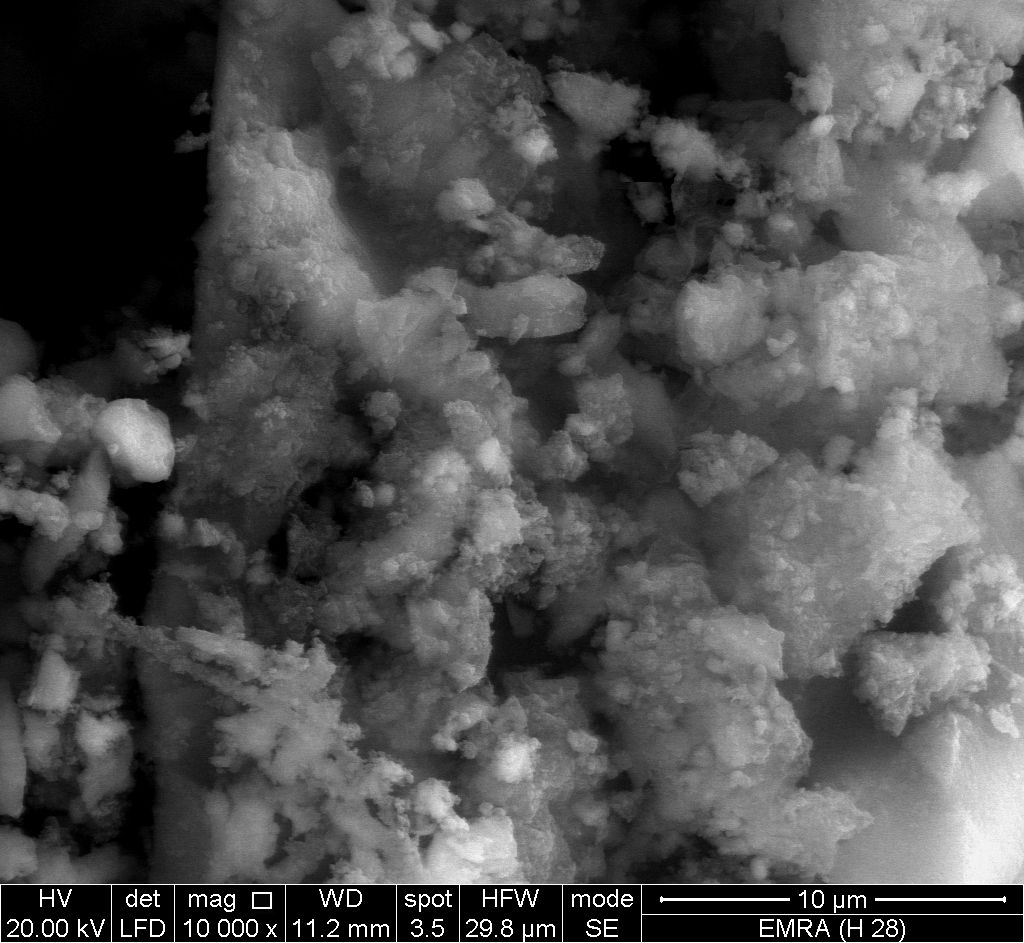 | Figure 5. SEM image of SnP/polyaniline |
3.5.2. FT-IR of SnP/Polyaniline Composite
- Figure (6) shows FT-IR spectrum glassy SnP/ polyaniline composite. Broad band centered around ~3425 cm-1, is due to OH groups symmetric stretching of H2O super imposed with the N–H stretching of aromatic amines at the range 3749-2854 cm-1. Very small band around ~1641 cm-1 is related to H-O-H bending, and sharp broad band 1020 cm-1 corresponds to phosphate groups vibration. Small band at ~2924 cm-1 corresponds to C-H bonds, the presence of bands at 1546, 1516, 1457, and 1252 cm-1 are related to the non-symmetric C6 ring stretching modes. However the higher frequency vibration at ~1516 cm−1 has a major contribution from the quinoid rings C=C stretching vibration of quinoid ring, and depicts the presence of benzenoid rings also (C=C stretching vibration of benzenoid ring). Thus FTIR spectrum confirms the formation of the composite.
3.6. SnP/Polypyrrole Composite
- It was found when pyrrole in ethanol solution was added to slurry aqueous solution of glassy tin phosphate, with stirring, then followed by the addition of the oxidant FeCl3, to give black color by the end of the reaction period. The resultant SnP/ polypyrrole composite was characterized by elemental (C, H, N) analysis, FT-IR spectroscopy and SEM. From elemental (C, H, N) analysis. The amount of polypyrrole present in the composite was 36.48% % in wt.
 | Figure 6. FT-IR of SnP/polyaniline |
3.6.1. SEM of SnP/Polypyrrole Composite
- Figure (7) shows SEM image for SnP/polypyrrole composite reveal distribution of the polymer on the inorganic matrix.
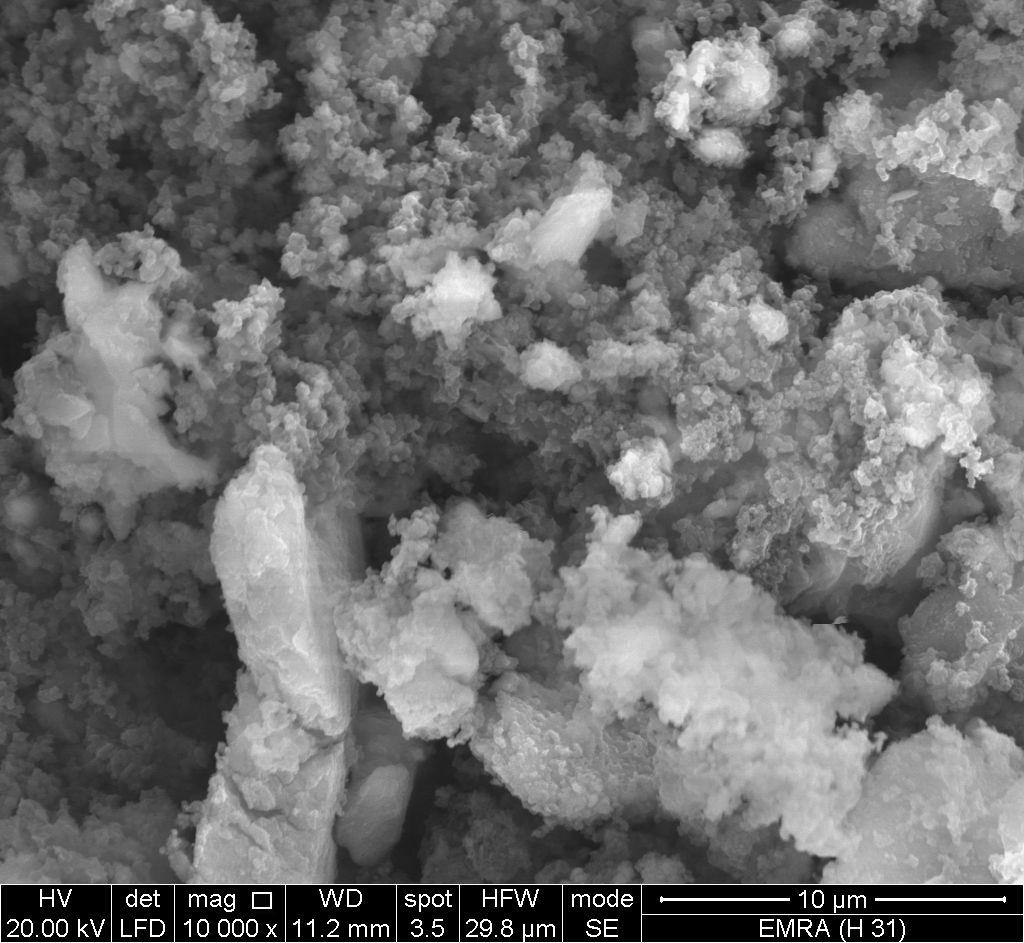 | Figure 7. SEM image of SnP/polypyrrole |
3.6.2. FTIR of SnP/Polypyrrole Composite
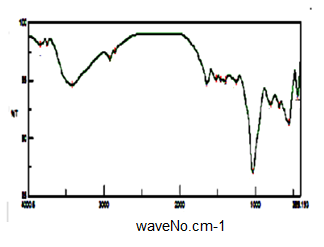
- Figure (8) shows FT-IR spectrum glassy SnP/polypyrrole composite. Broad band centered around ~3901 cm-1, is due to OH groups symmetric stretching of H2O super imposed with the N–H stretching of aromatic amines at the range 3763-2923 cm-1. Very small band at 1626 cm-1 is related to H-O-H bending, and sharp broad band, centered at 1036 cm-1 corresponds to phosphate groups vibration. Small band at ~2923 cm-1 corresponds to C-H bonds, The presence of bands at 1458, 1290, 1109, and 933.4 cm-1 are related to (C=C stretching, stretching and C–N bond stretching) of polypyrrole. Thus FT-IR spectra confirms the formation of the composite.
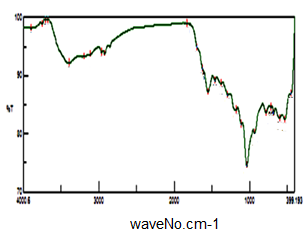 | Figure 8. FT-IR of SnP/polypyrrole |
3.7. SnP/Polyindole Composite
- It was found when indole in ethanol solution was added to slurry aqueous solution of glassy tin phosphate, Sn(HPO4)2.3H2O, with stirring, then followed by the addition of the oxidant FeCl3 to give brown color by the end of the reaction period. The resultant SnP/polyindole composite was characterized by elemental (C, H, N) analysis FT-IR spectroscopy and SEM. From elemental (C, H, N) analysis, the amount of organic material present in the SnP/polyindole composite was found to be 15% in wt.
3.7.1. SEM of SnP/Polyindole Composite
- Figure (9) shows SEM image for glassy, SnP/ polyindole composite, reveal a distribution of the polymer on the inorganic matrix.
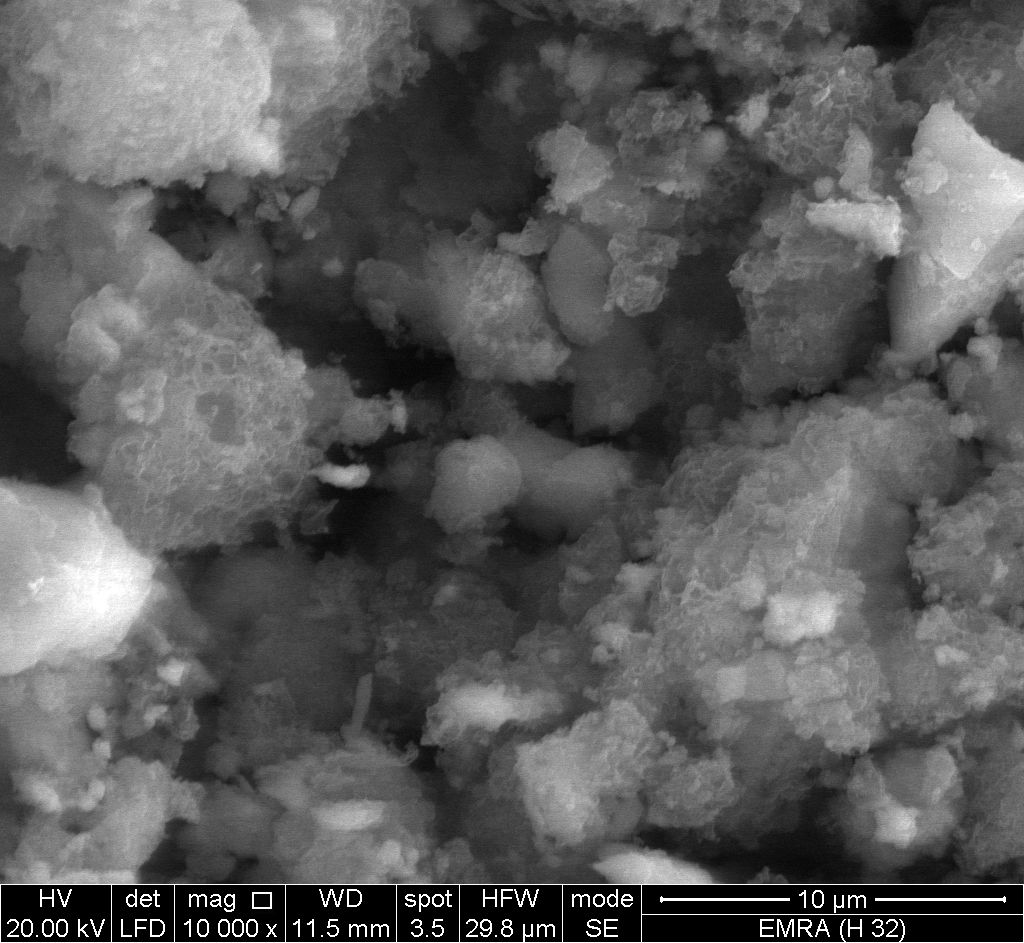 | Figure 9. SEM image of SnP/polyindole |
3.7.2. FTIR of SnP/Polyindole Composite
- Figure (10) show FT-IR spectrum glassy SnP/polyindole composite. Broad band at the range 3747-3000 centered around ~3397 cm-1, is due to OH groups symmetric stretching of H2O super imposed with the N–H stretching of aromatic amines at the range 3747-2455 cm-1. Very small band around ~1623 cm-1 is related to H-O-H bending, Small bands at 2923 and 2855 cm-1 could be attributed to N-H stretching. Bands in the region 1572-1332 cm-1 are related to stretching C-C bonds characteristic of indole unites C-H (aromatic) stretching, C=C stretching (between two indole units), sharp broad band centered at 1033 cm-1 is corresponds to phosphate groups vibration.
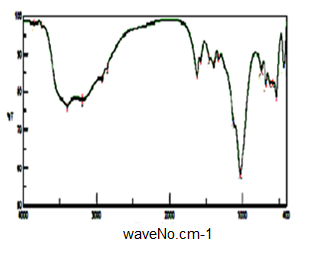 | Figure 10. FT-IR of SnP/polyindole |
3.8. SnP/polyindole-co-polyaniline Composite
- It was found when solution of mixture of indole and aniline was added to slurry aqueous solution of glassy tin phosphate, Sn(HPO4)2.2.78H2O, with stirring , then followed by the addition of the oxidant FeCl3 to give brown color by the end of the reaction period. The resultant SnP/ polyindole co-polyaniline composite was characterized by elemental (C, H, N) analysis FT-IR spectroscopy and SEM. From elemental (C, H, N) analysis. The amount of organic material present in the SnP/polyindole-co-polyaniline composite was 3.837% in wt, where PIn = 0.54% in wt, Pani = 3.29% in wt.
3.8.1. SEM of SnP/Polyindole-co-polyaniline Composite
- Figure (11) shows SEM image for glassy, SnP/ polyindole-co-polyaniline composite, reveal a distribution of the copolymer on the inorganic matrix.
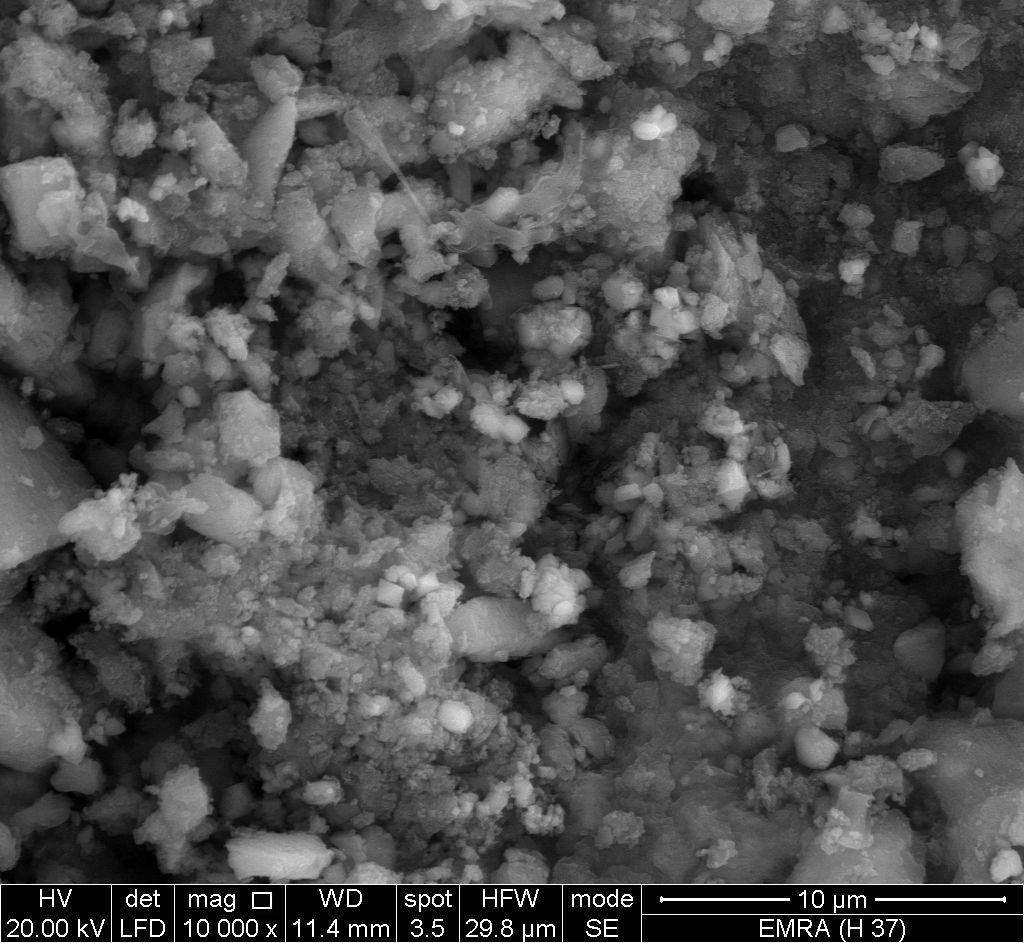 | Figure 11. SEM image of SnP/polyindole-co-polyaniline |
3.8.2. FTIR of SnP/polyindole-co-polyaniline Composite
- Figure (12) shows FT-IR spectrum of glassy SnP/polyindole-co-polyaniline composite. Broad band at the range 3763-2980 cm-1, centered around 3411 cm-1, is due to OH groups symmetric stretching of H2O super imposed with the N–H stretching of aromatic amines. medium band around 1637cm-1 is related to H-O-H bending. Small band at ~2924 cm-1 corresponds to C-H bonds. The presence of bands in the range of 1543-1400cm−1 assigned to the non-symmetric C6 ring stretching modes, However the higher frequency vibration at ~1543 cm−1 has major contribution from the quinoid rings C=C stretching vibration of quinoid ring, and depicts the presence of benzenoid rings also C=C stretching vibration of benzenoid ring. Bands in the range of 1543-1400cm−1 can also related to stretching C-C bonds characteristic of indole unites, C-H (aromatic) stretching, C=C stretching (between two indole units). Sharp broad band, centered at 1036 cm-1 corresponds to phosphate groups vibration.
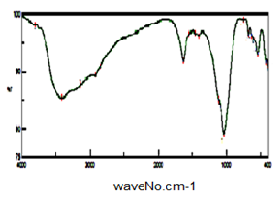 | Figure 12. FT-IR of SnP/polyindole-co-polyaniline |
3.9. SnP/polyindole-co-polypyrrole Composite
- It was found when solution of mixture of indole and pyrrole was added to slurry aqueous solution of glassy tin phosphate, Sn(HPO4)2.2.78H2O, with stirring, then followed by the addition of the oxidant FeCl3 to give black color by the end of the reaction period. The resultant SnP/polyindole-co-polypyrrole composite was characterized by elemental (C, H, N) analysis FT-IR spectroscopy and SEM. From elemental (C, H, N) analysis. The amount of organic material present in the SnP/polyindole-co-polypyrrole composite was 41.725% in wt, where PIn = 11.803% in wt, PPy = 30.023% in wt.
3.9.1. SEM of SnP/polyindole-co-polypyrrole Composite
- Figure (13) shows SEM image for glassy, SnP/ polyindole-co-polypyrrole composite, reveal a distribution of the copolymer on the inorganic matrix.
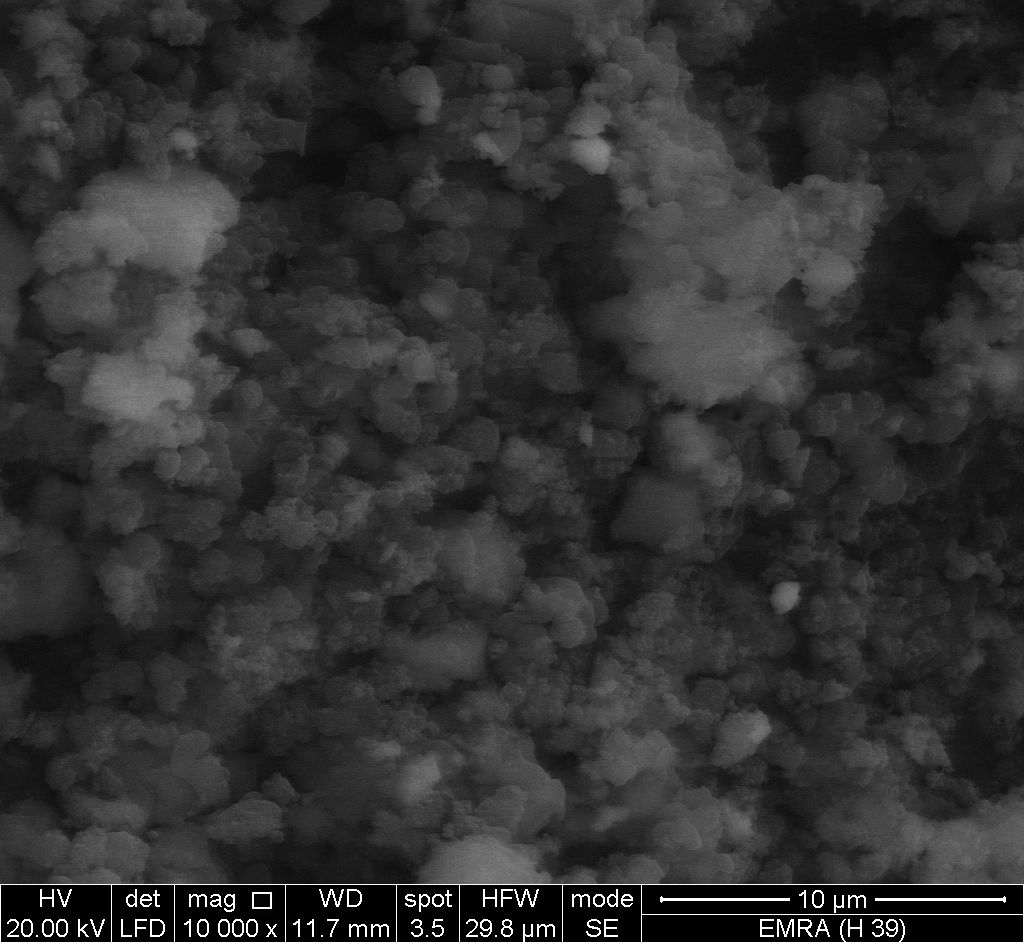 | Figure 13. SEM image of SnP/polyindole-co-polypyrrole |
3.9.2. FTIR of SnP/polyindole-co-polpyrrole Composite
- Figure (14) shows FT-IR spectrum glassy SnP/polyindole-co-polypyrrole composite. Broad band at the range 3746-2960 cm-1 centered at 3300 cm-1, is due to the characteristic stretching vibration OH groups symmetric of H2O, superimposed with N-H stretching of aromatic amines. Small bands at the range 2960-2853cm-1 could be attributed to N-H stretching. Bands in the region 1571-1333cm-1 are related to C=C stretching, C-N stretching (between two indole units), also correspond to the C=C stretching, C=N and C–N bonds of polypyrrole unites.
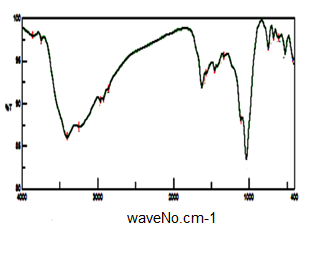 | Figure 14. FT-IR of SnP/polyindole-co-polypyrrole |
4. Conclusions
- Glassy tin phosphate, Sn(HPO4)2.2.78H2O(SnP), was prepared and characterized. SnP/polyindole-, polyaniline-, polypyrrole composites and SnP/ polyindole-co-polyaniline-, polyindole-co-polypyrrole composites were synthesized via in-situ chemical polymerization process, where the oxidant was FeCl3. The resultant composites were characterized by elemental (C,H,N) analysis, FT-IR spectroscopy, and scanning electron microscopy(SEM). SEM images of the resultant composites reveal distribution of the polymers and co-polymers on the inorganic matrix. From elemental (C,H,N) analysis the amount of organic material present in SnP/PIn composite = 15.0% in wt, SnP/Pani composite = 3.298% in wt, SnP/PPy composite = 36.48% in wt., respectively. The amount of organic material present in SnP/PIn-co-Pani = 3.837, where (PIn = 0.54 % in wt, Pani = 3.29 % in wt). Organic material present in SnP/PIn-co-PPy found to be 41.725% in wt, where (PIn = 11.803% in wt, PPy = 30.023% in wt). The FT-IR spectra supports the formation of the resultant composites. Composites of SnP/polyindole-co-polyaniline and SnP/polyindole-co-polypyrrole are a copolymers not a mixture of polymers.These composites can be considered as novel conducting inorganic-organic composites, ion exchangers and solid acid catalysts.
ACKNOWLEDGEMENTS
- To Department of Chemistry, Faculty of Science, Tripoli University for the supporting of this research. To geologist Adel Bayuomi, mineral resources Centre, Egypt, for providing facilities for SEM, elemental (C,H,N) analysis and FT-IR spectroscopy.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML