-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2016; 6(3): 80-85
doi:10.5923/j.chemistry.20160603.03

Determination of Functional Properties of Acid Treated Acha (Digitaria Exilis) Starch Using 36% HCL
Shehu Isah1, A. A. Oshodi2, V. N. Atasie1
1Bells University of Technology, Ota, Nigeria
2Federal University of Technology, Akure, Nigeria
Correspondence to: Shehu Isah, Bells University of Technology, Ota, Nigeria.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Acid treatment of acha (Digitaria Exilis) starch was carried out using 36% hydrochloric acid in an ethanolic medium. The functional properties of chemically modified sample and the native starch were evaluated: including solubility, water/oil absorption capacity, foam capacity, emulsion capacity, bulk density, pasting property, least gelation concentration (LGC) and starch granule morphology. The pasting viscosity was significantly reduced (50% reduction) on acid treated sample and the least gelation concentration also increased. The emulsion capacity and solubility were increased following acid treatment. But the foam capacity, water / oil absorption capacity and bulk density were reduced on the acid treated sample. Starch granule morphology was investigated using scanning electron microscope (SEM). Increased porous region was observed with the chemically modified sample. The infrared spectra showed similar peaks.Thus, acid treated acha starch possesses potential applications in preparation of low viscosity starch in food, paper and textile industries such as instant soups, stew, gravies sauce, custard, canned food, paper sizing, adhesives, encapsulation of flavor , fats and oil. It also showed promise as a good emulsifying agent.
Keywords: Chemical modification, Granule morphology, Retrogradation and seneresis, Least gelation concentration
Cite this paper: Shehu Isah, A. A. Oshodi, V. N. Atasie, Determination of Functional Properties of Acid Treated Acha (Digitaria Exilis) Starch Using 36% HCL, American Journal of Chemistry, Vol. 6 No. 3, 2016, pp. 80-85. doi: 10.5923/j.chemistry.20160603.03.
1. Introduction
- In several industrial applications a high content of starch is desired for the starch to form a gel as seen in wine gums and liquorice. When native starch is used as the gelling agent the paste would become too viscous during heating as a very high starch concentration is required to form a gel. In such application acid treated starches are utilized.Acid treated starches have a low viscosity. In many applications, for example in instant soups, thin boiling starches are often used as filler without any specific technical function. Acid treated starches have been degraded. The most common methods of degrading the starches are oxidation, acid treatment, and enzymatic. Acid treated starches are degraded starches for purpose of low viscosity typical for certain application such as filler in instant soups. When starch is degraded through acid treatment, the glycosidic starch chains are broken almost the way they are when oxidation is used. The difference is that the starch is not stabilized as it is with the oxidation. This shows in a much higher end-viscosity than with oxidation.The oxidized starch has a much lower end-viscosity than the acid hydrolyzed starch, which is due to the carboxylic acid groups stabilizing the starch in oxidized chemical modification. Acetylation of oxidized starch improves its stability. When starch is completely hydrolyzed, products such as malto-dextrin, corn syrup, glucose syrup and high glucose syrup have a wide application in the food, textile, brewing, and pharmaceutical industries [1]. These products are mainly derived from corn, barley and potato starch. In Malaysia, sago starch is considered as one of the most important sources of starch. It was reported [2] that about 60 million tonnes of sago starch extracted from sago palms are produced per annum in South-east Asia. However, the raw sago starch exists as large granules with compact crystalline structure. As a result, the enzyme reaction rate and yield of products from raw sago starch was reported to be too low for industrial applications [2].Bioconversion of sago starch was limited by the resistance of the raw granule to enzymatic hydrolysis. It has been reported that treatment of raw starches with acid at below its gelatinization temperature would enhance its digestibility by enzymes. Therefore, in order to increase the susceptibility of raw sago starch to enzymatic hydrolysis and improve glucose production, sago starch was treated with acid below its gelatinization temperature. The effect of acid treatment on the production of glucose using raw starch as the substrate was studied [3].Swelling power, solubility and water binding capacity of starches decreased following acid modification. The morphological properties revealed hydrolysis of starch granules is due to attack of acid on amorphous regions, which come in contact with the acid leading to fusion of granules. After modification, starch granules tended to appear fused and less smooth than the native starch granules. The acid modified starches reported slightly higher pasting temperature compared to their native counterparts. The acid modification reduced thickening ability of starches that is based on swollen capacity of undamaged granules as revealed by pasting properties. Most starch types consist of granules in which two types of glucose polymers are present. These are amylose (15-35 wt. % on dry substance) and amylopectin (65-85 wt. % on dry substance). Amylose consists of unbranched or slightly branched molecules having an average degree of polymerization of 1000 to 5000, depending on the starch type. Amylopectin consists of very large, highly branched molecules having an average degree of polymerization of 1, 000, 000 or more. The commercially most important starch types (maize starch, potato starch, wheat starch and tapioca starch) contain 15 to 30 wt. % amylose. Of some cereal types, such as barley, maize, millet, wheat, rice and sorghum, there are varieties of which the starch granules nearly completely consist of amylopectin. Calculated as weight percent on dry substance, these starch granules contain more than 95%, and usually more than 98% amylopectin. The amylose content of these cereal starch granules is thus less than 5%, and usually less than 2%. The above cereal varieties are referred to as waxy cereal grains. For instance, potato starch granules isolated from potato tubers usually contain about 20% amylose and 80% amylopectin (wt. % on dry substance). During the past 10 years, however, successful efforts have been made to cultivate by genetic modification potato plants which, in the potato tubers, form starch granules consisting for more than 95 wt. % (on dry substance) of amylopectin. It has even been found feasible to produce potato tubers comprising substantially only amylopectin.
2. Materials and Methods
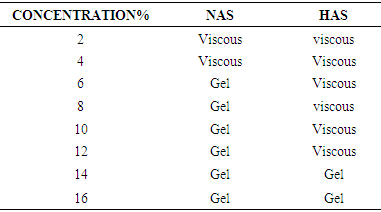
- (a) Sample collection White Acha grains (digitaria exilis) were purchased at a local market in Kubwa, Abuja, Nigeria. The sample was verified by the biological sciences department of Bells University of Technology, Ota, Ogun state, Nigeria.Preparation of starch slurryThe methods already described [4] with some modification was used. 2kg of winnowed D exillis was steeped in 5 liters of distilled water for 24h at 28°C, after which the solution was discarded and swollen grains, washed with water. The sample was then blended using a domestic milling machine. The slurry obtained was suspended in 5L of distilled water. The slurry obtained was centrifuged at 4500 r.p.m for 30 minutes. The starch obtained was reslurried after centrifugation in 5L of distilled water. The protein was separated from starch using 0.1M NaOH in adjusting pH to between 8.5 and 9.0. An emulsion layer of denatured protein formed was discarded. The process was repeated for the starch slurry until the emulsion layer became less visible. The starch slurry was finally washed with acetone and air dried for 24hours at 28°C.Acid treatment of starch The procedure described [5] with some modification was used. 25g of Acha starch was suspended in 100ml of ethanol in a 500ml conical flask. To the starch solution was added 20ml of 36% HCl and reaction allowed to proceed for 1 hour at 45°C in a shaking water bath. The reaction was then stopped by neutralizing the solution media with 1M NaOH. The slurry was transferred into 50ml centrifuge tube and centrifuged at 3500rpm for 5 minutes. The supernatant collected and the precipitate washed with 50% ethanol until neutral to litmus. The acid treated starch was filtered using Whatman No. 1 filter paper and dried in an oven at 40°C, weighed at room temperature.Determination of physicochemical propertiesSolubility:The native starch and modified starch samples (2g each) were suspended in 20ml of distilled water. Then heated to 70°C for 30 minutes with continuous shaking. The mixture was then centrifuged at 4000 rpm for 15 minutes. An aliquot of supernatant (5ml) was evaporated at 105°C and weighed. The solubility of starch is the ratio in mass (g) of the dried supernatant to the initial mass (g) of dried starch [7].(b) Water and Oil absorption capacity1g of native and modified starch were weighed into test tubes. 10ml of distilled water (and 10ml of groundnut oil in the second test tube) were added, and then heated in a water bath at 60°C for 30 minutes. The starch slurry was centrifuged at 1000rpm for 15 minutes and the supernatant carefully decanted and the weight of the starch paste taken. WAC/OAC= weight of starch paste/weight of dry starch sample [7].(c) Bulk densities of native and modified starch2 grams each of native Acha starch and the modified starch were placed in a 10ml measuring cylinder and the volume occupied by the sample without tapping recorded. The bulk density is the ratio of the weight to volume occupied [7].(d) Least gelation concentration of starchThe method described [7] with some modification was used. Eight samples each for native and modified starches (1-16% w/v) were prepared in test tubes with 5ml of distilled water. The starch solutions were mixed using magnetic stirrer for 5 minutes and heated for 30 minutes at 80°C in a water bath followed by rapid cooling under running cold water. Further cool at 4°C for 2 hours. Least gelation concentration was determined as that conc. when the samples from the inverted test tube did not fall down or slip.(e) Pasting properties of the starchPasting properties of the native starch and modified sample was carried out using Brookfield viscometer. Readings were taken after 10 revolutions each.(f) Foam capacity of starch2 grams of native Acha starch and each of modified starch were homogenized in 100ml of distilled water using a magnetic stirrer for 5 minutes. The homogenate was poured into a 250ml measuring cylinder and the volume occupied was recorded after 30 seconds. The foam capacity is expressed as the percent increase in volume [7].(g) Emulsion capacity of starch2g of native and modified acha starch were dispersed in 25ml of distilled water using a magnetic stirrer for 30 seconds. After complete dispersion, 25ml of vegetable oil (groundnut oil) was added gradually and the mixing continued for another 30 seconds. Then centrifuged at 1600rpm for 5 minutes. The volume of oil separated from the sample was read directly from the tube. Emulsion capacity is the amount of oil emulsified and held per gram of sample [7].(h) Starch Granules MorphologyThe starch granule Morphology of both the native starch and modified starch were obtained using scanning electron microscopy (SEM) at 25 kev accelerating voltage and 2500 magnification each.
3. Results and Discussions
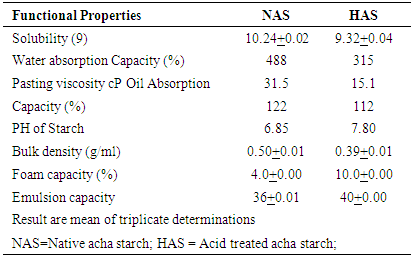
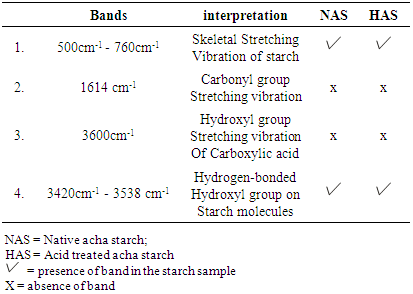
- Physico chemical propertiesSolubilityThe results of solubility of native acha starch (NAS) and acid treated starch (HAS) are shown on table 1. The solubility expressed as gram per 100 gram of starch (g/100g) increased from 10.24 value observed with the native acha starch to 11.50g for the modified starch. Solubility increased following acid treatment modification. This increase in solubility following acid treatment may be attributed to increase in the hydroxyl groups resulting from the break in glycosidic bond linkages and reduction in molecular weight distributions.
|
|
|
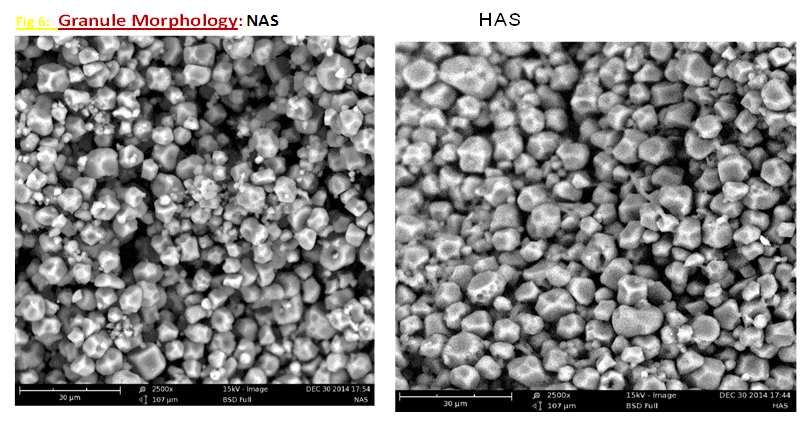 | Figure 1. Comparison of Granule morphology NAS vs HAS |
 | Figure 2. Infra red spectra of NAS sample |
 | Figure 3. Infra red spectra of HAS Sample |
4. Conclusions
- Chemical modifications of starch obtained from acha grain were successfully carried out. Physicochemical properties of native acha starch (NAS) and modified derivative (HAS) were determined. Chemical modifications enhanced emulsion capacity. Water and oil absorption capacities, rheological properties expressed as paste viscosities were lowered on modified samples. Potential applications of modified acha starch include good emulsifying agent, starch thickened sauces, soups, paper binding and pharmaceutical drug carriers and disintegrants. Modification improved stability and retro gradation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML