-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
American Journal of Chemistry
p-ISSN: 2165-8749 e-ISSN: 2165-8781
2016; 6(3): 74-79
doi:10.5923/j.chemistry.20160603.02

Surface Modification of Activated Carbon for Improved Iodine and Carbon Tetrachloride Adsorption
O. A. Babatunde1, S. Garba1, Z. N. Ali2
1Chemistry Department Nigerian Defence Academy, Kaduna, Nigeria
2Applied Science Department Kaduna Polytechnic, Kaduna, Nigeria
Correspondence to: Z. N. Ali, Applied Science Department Kaduna Polytechnic, Kaduna, Nigeria.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Activated carbon produced from Cocosnucifera,was modified using nitric acid, acetic acid, ascorbic acid and sodium hydroxide. The effects of this chemical modification were studied using iodine and carbon tetrachloride adsorption. Characterization of the activated carbons using scanning electron microscopy and FTIR analysis showed a greater development of macro porosity obtained by the modified activated carbons and the FTIR spectra displayed bands confirming the presence of carboxyl, hydroxyl, and carbonyl functional groups. The predicted influence of chemical modification on activated carbon surface for iodine and carbon tetrachloride uptake and adsorption isotherm study from the adsorption models, agreed satisfactorily with the experimental values.
Keywords: Cocos nucifera, Activation, Modification, I2 & CCl4 adsorption
Cite this paper: O. A. Babatunde, S. Garba, Z. N. Ali, Surface Modification of Activated Carbon for Improved Iodine and Carbon Tetrachloride Adsorption, American Journal of Chemistry, Vol. 6 No. 3, 2016, pp. 74-79. doi: 10.5923/j.chemistry.20160603.02.
Article Outline
1. Introduction
- Activated carbon is a highly micro porous material that has been employed as one of the main adsorbents in removal processes of heavy metals, dyes, taste and odour compounds, pesticides and natural organic chemicals such as humic and fulvic acids from water [9]. Activated carbon is not only used for adsorption of substances from liquid or gaseous streams; some of its other uses include corn and sugar refining, as catalysts or catalyst support, in electroplating or as electrodes in batteries [7]. Activated carbons were formerly produced from non-renewable and relatively high cost materials such as coal which is a cause for concern in pollution control applications [19]. However in recent years, many researchers have produced activated carbons using renewable and cheaper precursors which are mainly industrial and agricultural by-products [7; 21], some of which include coconut shell [26], oil-palm stone [27], oil bean pod [28], banana bark [29], bagasse, sorghum and millet straws [30], apricot stone [31]. The activated carbons were mostly prepared chemically and had surface areas ranging from 630.8 m2/g - 1387 m2/g; and were tested for adsorption of various substances which include SO2, NH3, Cd (II) ions, methylene blue, iodine and gold ions. The specific adsorption characteristics of an activated carbon is however strongly de- pendent on the composition of the surface functional groups which are incorporated into the activated carbon either by physical or chemical treatment. Attempts have been made by many researchers to modify the surface structures of activated carbons with substances having oxygen-containing functional groups such as acids and/or bases e.g. nitric acid [32], tannic acid [33], citric acid [34], sodium hydroxide [35], ammonia [34]. Other modifying techniques include using ozone, CO2, NO2, and plasma treatment [36]; [37]. The objectives of all these treatments were to modify the pore size, control the pore size distribution and modify the activated carbon’s polarity in order to enhance its selective chelating ability. Iodine is a lustrous, violet-black, corrosive, poisonous halogen element having radioactive isotope. Iodine has been used to remove biological contamination from water [18], further controlled release of iodine is said to likely improve its effectiveness as water disinfectant, while Carbon tetrachloride is a volatile, clear, colourless, heavy liquid and also an ozone-depleting substance. Though, not flammable and not easily soluble in water [22]. It is however used in the production of refrigeration fluid, propellant for aerosol can, as a pesticide, a dry-cleaning fluid, e.t.c. [3]; but because of its harmful effects, these uses are now banned. However, both Iodine and Carbon tetra- chloride are used as test chemicals to measure the capacity of an activated carbon to adsorb chemical compounds either in liquid phase and or in gaseous phase., and according to their pore dimensions, the iodine molecule is mainly adsorbed in micropores; while carbon tetrachloride is adsorbed in mesopores or large micropores [13]. The iodine number (milligram of iodine adsorbed by one gram of carbon) which is a relative indicator of porosity of an activated carbon is usually used as an approximation of the surface area of an activated carbon.The aim of this research was to utilize coconut shell (a waste product) being discarded indiscriminately around for the preparation of activated carbon (a more value added product) and to also study the influence of chemical modification of the prepared activated carbon for enhanced iodine and carbon tetrachloride adsorption.
2. Experimental
2.1. Materials
- Coconut shells were obtained from the coconut sellers in Badarawa, Kaduna. Iodine (99.5%), Potassium iodide (99.9%), Sodium thiosulphate (99.5%), Potassium dichromate (99.5%), Phosphoric acid (85%) Nitric acid (69%), Acetic acid (99.7%) Ascorbic acid (94.5%), Hydrochloric acid (35.4%), Sodium hydroxide (99.0%) Formic acid (90 %) potassium hydroxide (90%) and Carbon tetrachloride (99.9%) were all of analytical grade and were purchased from BIJO Chemicals Ltd. Kaduna.
2.2. Methods
2.2.1. Carbonization and Activation Process
- The activated carbon was prepared by the two-step chemical activation process. The first step involves carbonization with 60% phosphoric acid solution and the second step involves activation using muffle furnace model SXL at 600°C for 1 hr and 1.0 M KOH [15]. The prepared activated carbon was first washed with water containing 0.15% formic acid and then washed severally with distilled water. They were filtered through a Buchner funnel dried at 120°C and then weighed to determine the activated carbon yield. It was then stored in plastic sample bottles to prevent adsorption of dust and other particles from the atmosphere [14].The activated carbon yield was calculated using Eq. 1
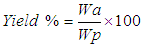 | (1) |
2.2.2. Modification of Activated Carbon
- To 10 g of the activated carbon sample, 100 cm3 of 2.0 M nitric acid was added and soaked for 24 hrs. The residual nitric acid was removed by soaking it in distilled water for 2 hrs and then washed several times with distilled water. The sample was then dried at 120°C, cooled in a desiccator and stored in plastic sample bottles; to obtained acetic acid, ascorbic acid and sodium hydroxide modified activated carbons, the experiment was repeated using 2.0 M CH3COOH, 2.0 M C6H8O6 and 2.0 M NaOH.
2.2.3. Characterization of Activated Carbon
- The surface physical morphology of the unmodified and modified activated carbons was observed using Phenom scanning electron microscope model proX, while FTIR analysis was also carried out on the same activated carbon samples to determine the surface functional groups using FTIR spectroscope (FTIR – 8400S, Shimadzu) and the spectra were recorded from 4000- 500 cm-1.
2.2.4. Iodine Number Test
- The iodine number was determined according to the ASTM D 4607-94 method [2]. This method is based upon a three-point isotherm. A 0.1 M standard iodine solution was added to three different weights of activated carbon samples in three 250 cm3 conical flasks. The experiment in-volved treating the activated carbon sample with 10.0 cm3 of 5% HCl. This mixture was boiled for 30 seconds and then cooled soon afterwards, 100 cm3 of 0.1 M iodine solution was added to the mixture and stirred for 30 seconds. The resulting solution was filtered and 50.0 cm3 of the filtrate was titrated with 0.1 M sodium thiosulphate using starch solution as indicator. The amount of iodine adsorbed per gram of carbon (iodine number) and the residual iodine concentration in the filtrate were calculated using Eq 2.
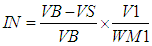 | (2) |
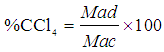 | (3) |
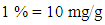
3. Result and Discussion
3.1. Activated Carbon Yield
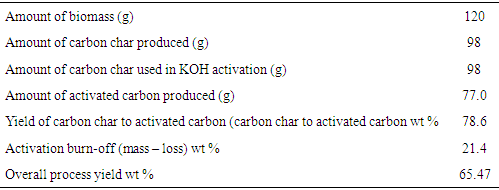
- The result of the activated carbon yield as presented in Table 1 showed a 78.6% yield from carbon char to activated carbon and an overall process yield of 65.47%. This result is similar to that reported by [16] for the production of activated carbon from chemically treated coconut shells but higher than that reported by [25] who obtained a yield of 40.62% for acid treated coconut shells.
|
3.2. Characterization of Unmodified and Modified Activated Carbon
- The scanning electron microscopic (SEM) images of the unmodified and modified activated carbons prepared under optimum conditions (600°C, 1 hr activation time and 2.0 M KOH) were presented in Figures 1 and 2. There was a general gradual increase in pore formation from unmodified to modified activated carbon. The SEM of unmodified coconut shell activated carbon (Figure 1) showed that the activation stage produced an extensive external surface with quite a number of irregular cracks and pores. Larger and well-developed pores were clearly found on the surface of the modified sample (Figure 2). These differences in the surface topology between the unmodified and modified activated carbons were as a result of modification process according to [20].
 | Figure 1. SEM of unmodified coconut shell activated carbon. Magnification: 1000× and scale: 80µm |
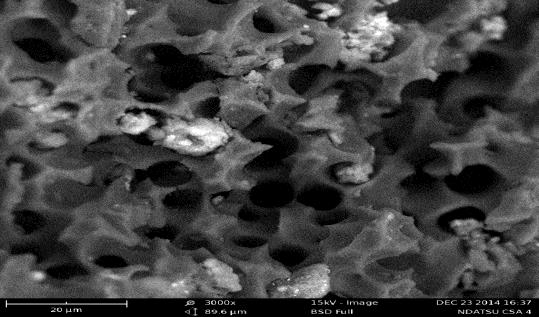 | Figure 2. SEM of modified coconut shell activated carbon. Magnification: 3000× and scale: 20µm |
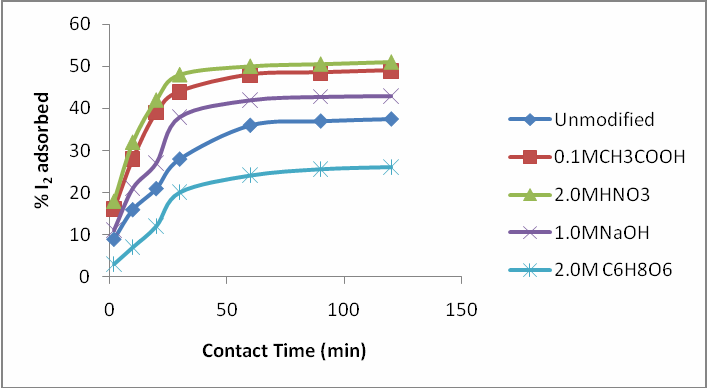 | Figure 3. The percent iodine adsorbed against contact time by unmodified activated carbon and the various modified activated carbons using 1.0 M KOH activating agent at 600°C |
3.3. FTIR Analysis
- The following functional groups were observed in the FTIR spectra for the unmodified and modified activated carbons. They include O-H, C=C, C-O, C-NO2 and C-H. The FTIR spectra obtained were in agreement with the result reported by [17] for activated carbon prepared from cherry stones. The band found in unmodified coconut shell activated carbon in the region of 3414. 12cm-1 are a result of an O-H stretching mode of hydroxyl group in alcohols, phenols or absorbed water. This was found to be slightly broader in the modified sample, e.g. for nitric acid modified coconut shell activated carbon (MCS) the band slightly shifted to 3472.95cm-1. The band at 1644.37cm-1 due to C=C stretching vibration in alkenes also occurred at 1642.44cm-1 for the modified activated carbon. The band in the region of 1378.18cm-1 that is due to the stretching vibration of the nitro group (NO2); which is of the same frequency band as NO3- in nitric acid [18] were only detected in the modified activated carbon.
3.4. Iodine Number
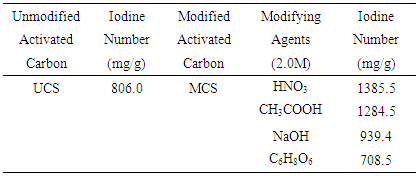
- Table 2 showed that the iodine number of unmodified activated carbon (UCS) was 806.0 mg/g. This result is similar to values reported by [24] for activated carbon produced by chemical activation of coconut shell with phosphoric acid. [10] However, reported lower iodine number values for activated carbon produced from waste date stones using H3PO4 as activation agent. The iodine numbers were found to increase tremendously after the modification process except for ascorbic acid modified activated carbon as presented in Table 2.
|
3.5. Adsorption Isotherms
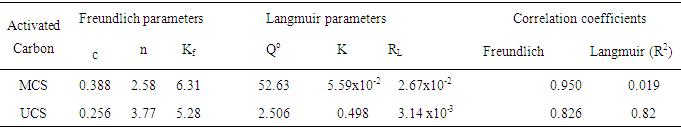
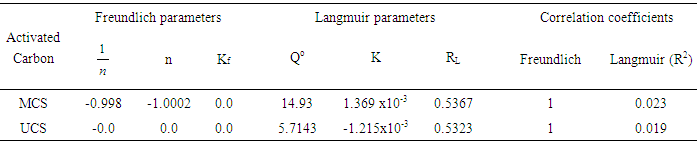
- The equilibrium data for iodine (Table 3) were well fitted into the Freundlich model with correlation coefficients (R2) of the modified activated carbon being higher (0.950) than those of unmodified activated carbons (0.826). Kf and
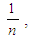 the intercept and the slope, which are parameters de-picting the adsorption capacity and adsorption intensity also indicated normal adsorption as reputed by [11]. The adsorption data for the unmodified activated carbon (UCS); fitted better into the Langmuir isotherm equation compared to the modified sample (MCS). The maximum mono-layer coverage (Qo) was also found to be higher in the modified activated carbon (52.63 mg/g) compared to the unmodified activated carbon (2.506 mg/g) with the highest value of 52.63 mg/g obtained by MCS. This result was lower than that reported by [6] for an equilibrium sorption study using modified rice husks activated carbon. [12], reported higher Qo values for the removal of Ni (II) from aqueous solutions. The equilibrium parameter (RL) which indicates the nature of the adsorption process [5] were greater than zero but less than 1 thereby indicating that all the adsorption processes were favourable [23].
the intercept and the slope, which are parameters de-picting the adsorption capacity and adsorption intensity also indicated normal adsorption as reputed by [11]. The adsorption data for the unmodified activated carbon (UCS); fitted better into the Langmuir isotherm equation compared to the modified sample (MCS). The maximum mono-layer coverage (Qo) was also found to be higher in the modified activated carbon (52.63 mg/g) compared to the unmodified activated carbon (2.506 mg/g) with the highest value of 52.63 mg/g obtained by MCS. This result was lower than that reported by [6] for an equilibrium sorption study using modified rice husks activated carbon. [12], reported higher Qo values for the removal of Ni (II) from aqueous solutions. The equilibrium parameter (RL) which indicates the nature of the adsorption process [5] were greater than zero but less than 1 thereby indicating that all the adsorption processes were favourable [23].
|
|
4. Conclusions
- The use of coconut shell, an agricultural by product, mainly used as fuel when burned to provide heat energy; to produce porous activated carbons offers a cheap, viable and renewable source of activated carbons. The modification of the activated carbon using nitric acid, produced activated carbons with desired physical properties and higher adsorptive capacities. Ethanoic acid and sodium hydroxide modified activated carbons also possess moderate physical properties and their adsorptive capacities were good though not as high as those modified with nitric acid. Ascorbic acid on the other hand, caused clogging up of the pores in the activated carbons and therefore reduced the activated carbon’s adsorptive capacity; between the two adsorption isotherms tested, the Freundlich isotherm gave the best fit for the adsorption of I2 and CCl4. The adsorption processes were also found to be favourable.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML